Endometrial Gene Expression Profiles During Ovarian Stimulation With Recombinant FSH With or Without the Addition of Recombinant LH in Genuine Poor Responders
This is a prospective randomized open-label cross-over study. Poor responder patients will undergo two ovarian stimulation cycles. One with recombinant follicle stimulation hormone (FSH), and an other stimulation with recombinant FSH and recombinant luteinizing hormone (LH). In both groups, a freeze-all strategy will be applied and an endometrial biopsy will be taken 7 days after final oocyte maturation trigger. Endometrial gene expression analysis will be performed on both biopsies. After completion of both treatment cycles, a frozen embryo transfer of a single embryo will be performed.
/ RecruitingNot Applicable Luteal Phase Ovarian Stimulation With Follitropin Delta and Dydrogesterone: a Randomized Cross Over Pilot Trial
The last decade has shown a progressive scientific interest for new strategies to improve the outcomes of controlled ovarian stimulation (COS).
Given the fact that interovulatory period has been described to have multiple waves of follicular recruitment, luteal phase ovarian stimulation (LPOS) has been proposed as new protocol for COS, with satisfactory ovarian response and pregnancy outcomes. On the other hand progestin-primed ovarian stimulation (PPOS) is today considered an innovative protocol aiming to achieve multi-follicle recruitment and block the luteinizing hormone (LH) surge through progesterone administration in place of the traditional down regulating or gonadotropin-releasing hormone (GnRH) antagonist. This protocol has been shown to be equally effective as LH suppression with GnRH antagonist reporting equivalent oocyte retrieval rates, endocrine profiles, viable embryo numbers, and pregnancy outcomes. Due to the feasibility and patients-friendly characteristics of PPOS in oocytes donors, the current study aims to investigate the impact on the number of cumulus-oocyte complexes (COCs) when a PPOS protocol is associated to both conventional follicular phase stimulation and LPOS for vitrification of oocytes in oocyte donors. Moreover, it aims to determine whether LPOS using PPOS protocol has comparable outcomes to conventional follicular phase stimulation with PPOS protocol, in oocyte donor patients.
/ RecruitingNot Applicable At High-risk for Pre-eclampsia After Assisted Reproductive Technology
The overarching goal of the project is to unravel PE etiopathogenesis in high-risk patients (PCOS patients and oocyte acceptors) after assisted reproductive technology (ART) to individualize prenatal care following ART and to determine potential targets for new PE prevention options decreasing the morbidity/mortality caused by this pathology. More specifically, the following objectives/work packages (WPs) are put forward:
WP1 - PRECONCEPTION: Identify preconceptional maternal characteristics associated with in-creased risk of PE in ART patients (1a) and investigate the potential role of the endometrium prior to pregnancy (1b).
WP2 - DURING PREGNANCY: Evaluate the Fetal Medicine Foundation's (FMF) first trimester PE screening in selected high-risk groups post ART to explore the clinical benefit in this specific context (2a) and investigate the association between parameters during the pregnancy and PE development post-ART.
WP3 - AT DELIVERY: Identifying placental molecular pathways associated with PE post-ART.
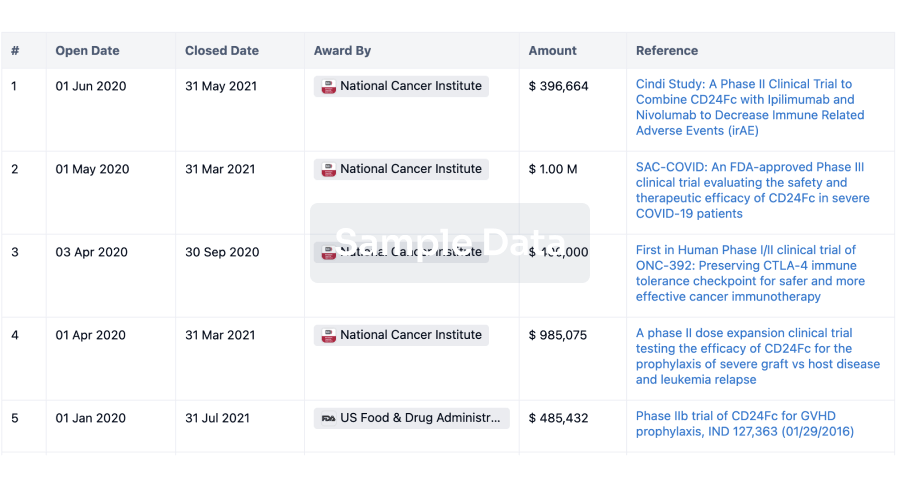
100 Clinical Results associated with Clean Room Garments PTY LTD
0 Patents (Medical) associated with Clean Room Garments PTY LTD
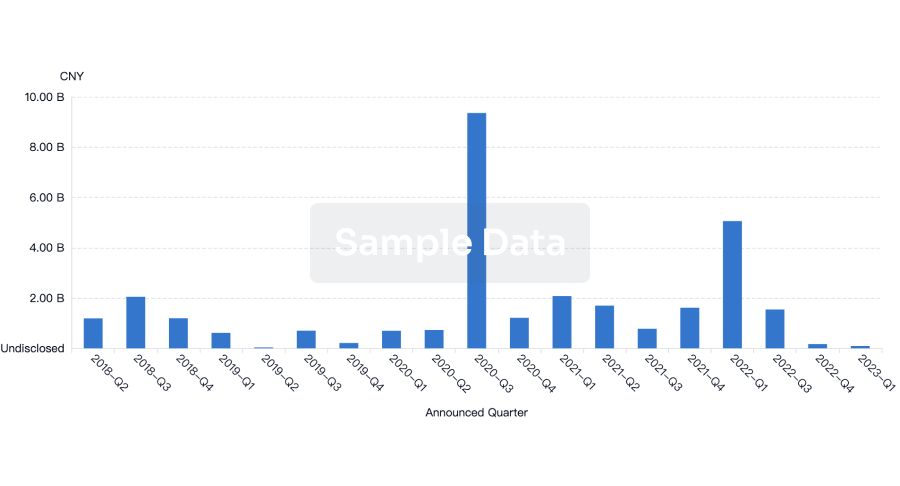
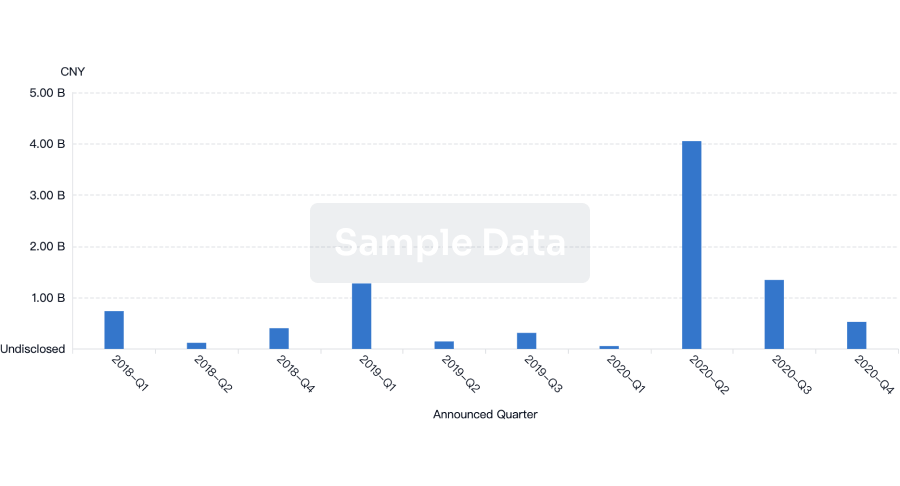
100 Deals associated with Clean Room Garments PTY LTD
100 Translational Medicine associated with Clean Room Garments PTY LTD