Intradiscal Gelified Ethanol in Refractory Cervical Discogenic Pain : a Interventional, Prospective, Multi-center, Open-label Study.
The purpose of this study is to assesse safety and efficacy of the Intradiscal Gelified Ethanol for treatment in refractory cervical discogenic pain.
Intradiscal Gelified Ethanol Versus Intradiscal Steroid in Refractory Lumbar Discogenic Pain: a Randomized Single-blind Study
DISCOGEL® is on the market since 2007. About 20,000 kits were sold to date (October 2017). The device re-obtained its CE mark in 2017.
A clinical evaluation was performed by bibliographic route in 2016. Clinical data on more than 600 patients treated by DISCOGEL® were analyzed. These data should be confirmed by monitoring on the long term, with a large cohort of patients, over a two-year follow-up period.
As part of the post-CE surveillance, the manufacturer GELSCOM is responsible of this "Post-Market Clinical Follow-up" (PMCF) study in accordance with Directive 93/42/EEC and MEDDEV guide 2.12/2, to assess the efficacy and the long-term safety of DISCOGEL®.
The study is comparative. The results will evaluate the performance and safety of the CE-marked medical device used in "real life", in comparison with a steroid infiltration, used according to its indication and to the current standards. It will include economic data. Patients and evaluators will be blinded. Both DISCOGEL® and HYDROCORTANCYL 2,5 POUR CENT are authorized products used according to their intended use.
This is an interventional, prospective, national, multi-center, comparative, randomized, single-blind (patient and evaluator) post-market clinical study. The primary objective is to compare the short-term efficacy profile of DISCOGEL® versus intradiscal steroid.
Non-inferiority Trial of Intradiscal Discogel® Versus Surgery in Sciatica Resistant to Conservative Treatment
Sciatica due to herniated disc is a major cause of disability in young adults. Surgery is the gold-standard and the only controlled treatment in case of failure of conservative treatment.
Percutaneous chemonucleolysis with Discogel® may be a valuable alternative to surgery.
In addition, Discogel® chemonucleolysis appears as a relatively innocuous technique which may avoid 2% complications after disc surgery and 5% repeated surgery (according to recent trials).
This will be the first randomized trial comparing Discogel® chemonucleolysis versus surgery in patients with sciatica due to lumbar disc herniation and unresponsive to conservative medical treatments (including epidural steroid injections) Our expectation is that Discogel® chemonucleolysis will avoid surgery in 80% of the patients.
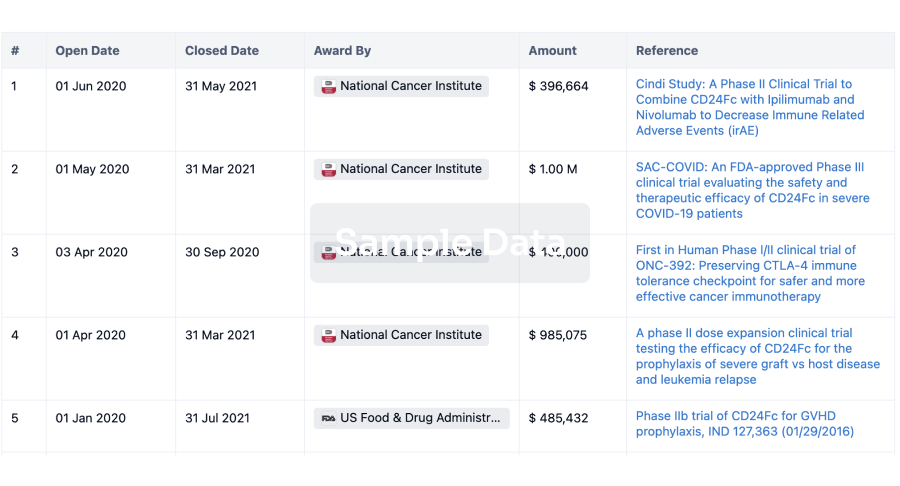
100 Clinical Results associated with Gelscom SAS
0 Patents (Medical) associated with Gelscom SAS
100 Deals associated with Gelscom SAS
100 Translational Medicine associated with Gelscom SAS