/ CompletedNot Applicable 健康志愿者餐后口服乙酰半胱氨酸泡腾片600mg生物等效性研究
[Translation] Bioequivalence study of oral administration of 600 mg acetylcysteine effervescent tablets after meals in healthy volunteers
通过对健康受试者进行餐后口服乙酰半胱氨酸泡腾片600mg研究,来评估受试制剂(健乔信元医药生技股份有限公司健乔厂生产的乙酰半胱氨酸泡腾片600mg)与参比制剂(意大利ZAMBON S.P.A公司生产的乙酰半胱氨酸泡腾片600mg,商品名:富露施)是否具有生物等效性,为该药临床应用的有效性和安全性提供依据,为生产与临床合理用药提供参考。
[Translation] The study was conducted on healthy subjects taking 600 mg of acetylcysteine effervescent tablets orally after meals to evaluate whether the test preparation (600 mg of acetylcysteine effervescent tablets produced by Jianqiao Plant of Jianqiao Xinyuan Pharmaceutical Biotechnology Co., Ltd.) and the reference preparation (600 mg of acetylcysteine effervescent tablets produced by ZAMBON S.P.A., Italy, trade name: Fluluciz) were bioequivalent, to provide a basis for the effectiveness and safety of the drug in clinical application, and to provide a reference for production and rational clinical use of the drug.
/ CompletedNot Applicable 健康志愿者空腹口服乙酰半胱氨酸泡腾片600mg生物等效性研究
[Translation] Bioequivalence study of 600 mg oral acetylcysteine effervescent tablets in healthy volunteers on an empty stomach
通过对健康受试者进行空腹口服乙酰半胱氨酸泡腾片600mg研究,来评估受试制剂(健乔信元医药生技股份有限公司健乔厂生产的乙酰半胱氨酸泡腾片600mg)与参比制剂(意大利ZAMBON S.P.A公司生产的乙酰半胱氨酸泡腾片600mg,商品名:富露施)是否具有生物等效性,为该药临床应用的有效性和安全性提供依据,为生产与临床合理用药提供参考。
[Translation] The study of oral administration of 600 mg of acetylcysteine effervescent tablets on fasting subjects was conducted to evaluate whether the test preparation (600 mg of acetylcysteine effervescent tablets produced by Jianqiao Plant of Jianqiao Xinyuan Pharmaceutical Biotechnology Co., Ltd.) and the reference preparation (600 mg of acetylcysteine effervescent tablets produced by ZAMBON S.P.A. of Italy, trade name: Flulucit) were bioequivalent, to provide a basis for the effectiveness and safety of the drug in clinical application, and to provide a reference for production and rational clinical use of the drug.
/ RecruitingNot Applicable 健康志愿者空腹/餐后口服乙酰半胱氨酸泡腾片600mg生物等效性研究
[Translation] Bioequivalence study of oral acetylcysteine effervescent tablets 600 mg on fasting or after meals in healthy volunteers
通过对健康受试者进行餐后口服乙酰半胱氨酸泡腾片600mg,来评估受试制剂(健乔信元医药生技股份有限公司健乔厂生产的乙酰半胱氨酸泡腾片600mg)与参比制剂(意大利ZAMBON S.P.A公司生产的乙酰半胱氨酸泡腾片600mg,商品名:富露施)是否具有生物等效性,为该药临床应用的有效性和安全性提供依据,为生产与临床合理用药提供参考。
[Translation] By taking 600 mg of acetylcysteine effervescent tablets orally after meals to healthy subjects, the bioequivalence of the test preparation (600 mg of acetylcysteine effervescent tablets produced by Jianqiao Plant of Jianqiao Xinyuan Pharmaceutical Biotechnology Co., Ltd.) and the reference preparation (600 mg of acetylcysteine effervescent tablets produced by ZAMBON S.P.A. of Italy, trade name: Flulucite) was evaluated, so as to provide a basis for the effectiveness and safety of the clinical application of the drug and a reference for the production and rational use of the drug in clinical practice.
100 Clinical Results associated with Beijing Aikang Weijian Medical Technology Co., Ltd.
0 Patents (Medical) associated with Beijing Aikang Weijian Medical Technology Co., Ltd.
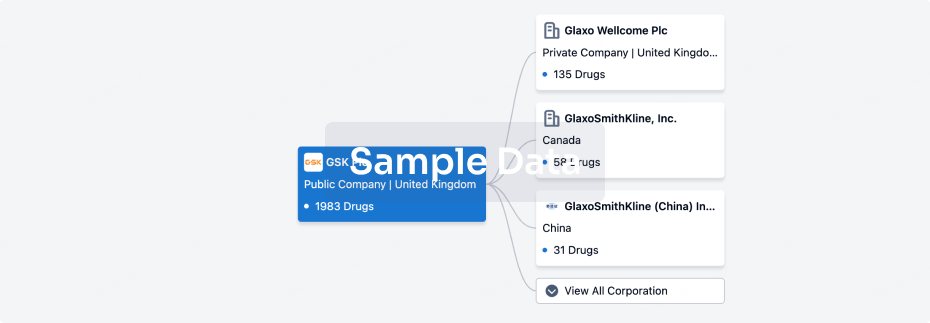
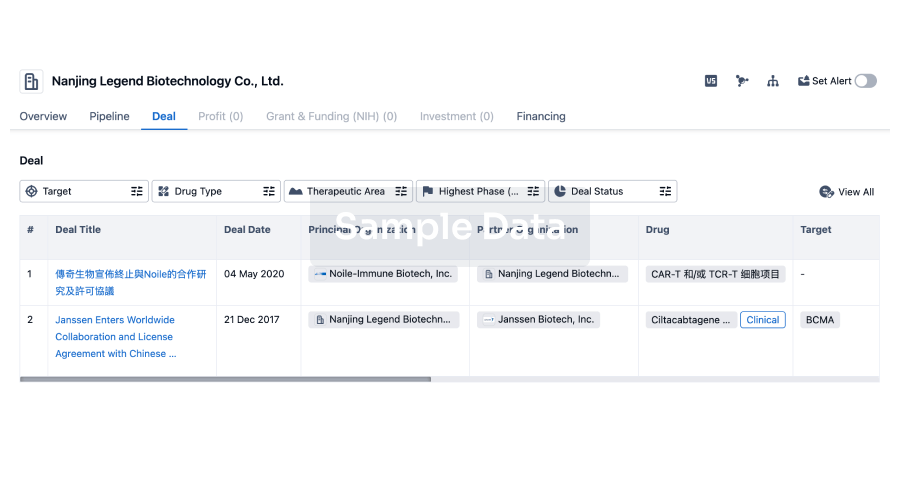
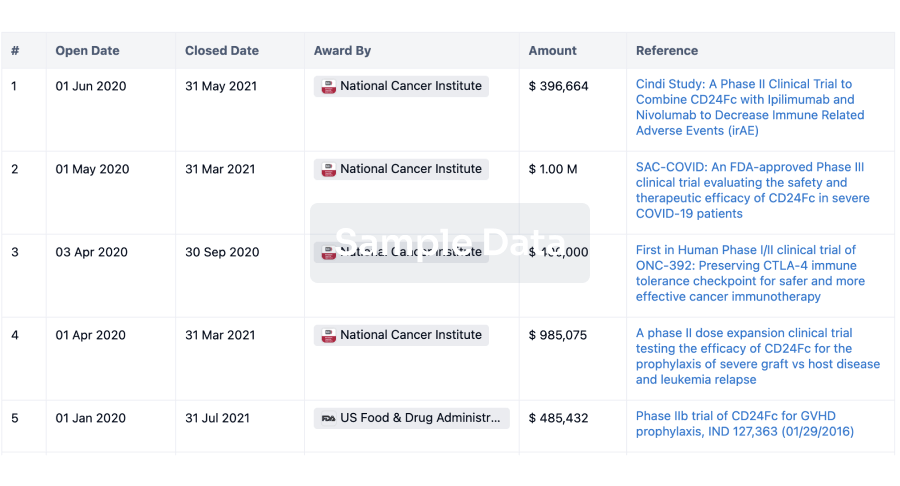
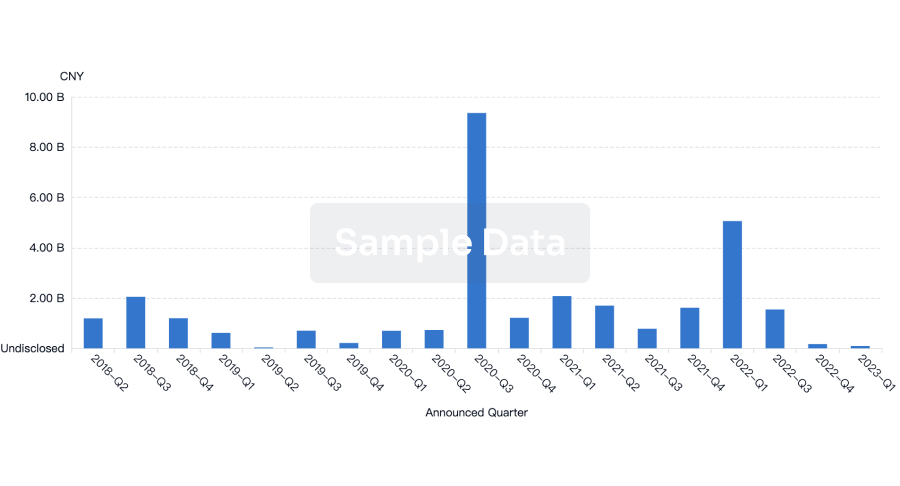
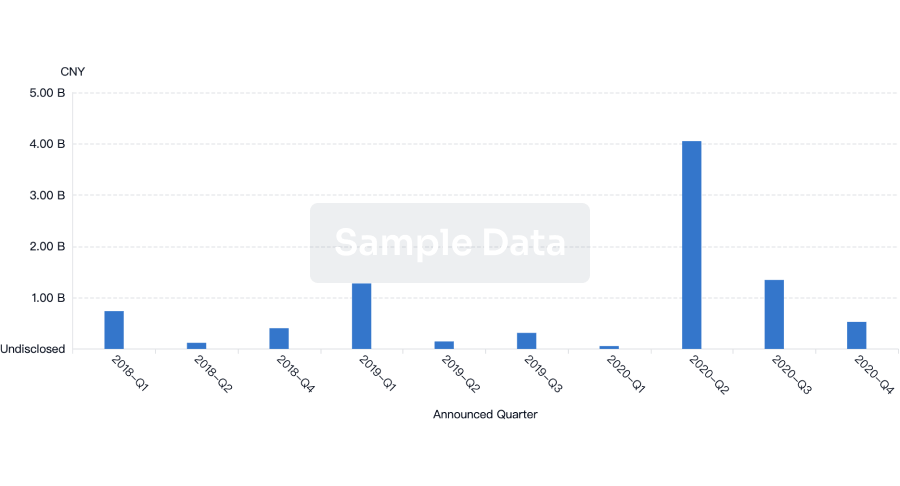
100 Deals associated with Beijing Aikang Weijian Medical Technology Co., Ltd.
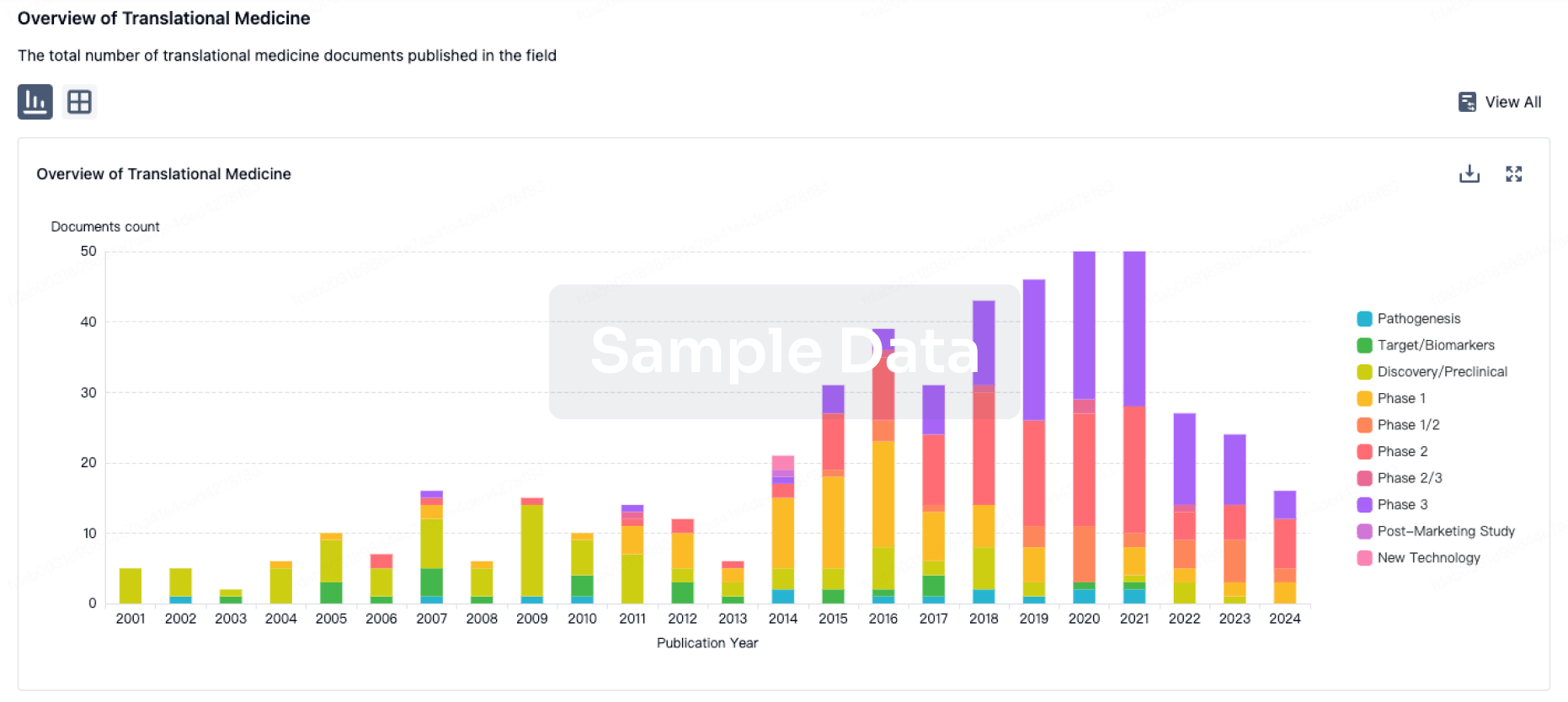
100 Translational Medicine associated with Beijing Aikang Weijian Medical Technology Co., Ltd.