/ Not yet recruitingNot Applicable [Translation] Bioequivalence study of milobarlin besylate tablets in human body
主要目的:以健康受试者为试验对象,采用开放、随机、两周期、双交叉试验设计,考察健康受试者空腹及餐后单次口服由南京万融健诚医药科技有限公司研制的苯磺酸米洛巴林片(规格15mg)与第一三共株式会社生产的苯磺酸米洛巴林片(参比制剂, TARLIGE ®,规格15mg)后米洛巴林的体内经时过程,估算其相关药代动力学参数及相对生物利用度,评价其空腹及餐后状态下的生物等效性,为受试制剂的生产注册申请提供依据。
次要目的:评价苯磺酸米洛巴林片受试制剂和参比制剂(商品名:TARLIGE®)在健康受试者中的安全性。
[Translation] Main purpose: Using healthy subjects as test subjects, an open, randomized, two-period, double-crossover design was used to investigate the in vivo time course of milobalin after a single oral administration of milobalin besylate tablets (specification 15 mg) developed by Nanjing Wanrong Jiancheng Pharmaceutical Technology Co., Ltd. and milobalin besylate tablets (reference preparation, TARLIGE ®, specification 15 mg) produced by Daiichi Sankyo Co., Ltd. on an empty stomach and after a meal, to estimate their relevant pharmacokinetic parameters and relative bioavailability, and to evaluate their bioequivalence in the empty stomach and after a meal, so as to provide a basis for the production registration application of the test preparation.
Secondary purpose: To evaluate the safety of the test preparation and reference preparation of milobalin besylate tablets (trade name: TARLIGE®) in healthy subjects.
/ CompletedNot Applicable [Translation] Postprandial bioequivalence study of melogabalin besylate tablets in healthy volunteers
主要目的:以健康受试者为试验对象,采用开放、随机、两周期、双交叉试验设计,考察健康受试者餐后单次口服由江苏万禾制药有限公司生产(南京万融健诚医药科技有限公司持证)的苯磺酸美洛加巴林片(受试制剂,规格:5mg)与第一三共株式会社生产的苯磺酸美洛加巴林片(参比制剂, TARLIGE ®,规格:5mg)后美洛加巴林的体内经时过程,估算其相关药代动力学参数及相对生物利用度,评价其餐后状态下的生物等效性,为受试制剂的生产注册申请提供依据。
次要目的:评价苯磺酸美洛加巴林片受试制剂和参比制剂(商品名:TARLIGE®)在健康受试者中的安全性。
[Translation] Main purpose: To use healthy subjects as test subjects, adopt an open, randomized, two-period, double-crossover design, to investigate the in vivo time course of melogabalin after a single oral administration of melogabalin besylate tablets (test preparation, specification: 5 mg) produced by Jiangsu Wanhe Pharmaceutical Co., Ltd. (certified by Nanjing Wanrong Jiancheng Pharmaceutical Technology Co., Ltd.) and melogabalin besylate tablets (reference preparation, TARLIGE ®, specification: 5 mg) produced by Daiichi Sankyo Co., Ltd. after meals, estimate their relevant pharmacokinetic parameters and relative bioavailability, evaluate their bioequivalence in the postprandial state, and provide a basis for the production registration application of the test preparation.
Secondary purpose: To evaluate the safety of the test preparation and reference preparation of melogabalin besylate tablets (trade name: TARLIGE®) in healthy subjects.
/ CompletedNot Applicable [Translation] Bioequivalence study of melogabalin besylate tablets in fasting volunteers
主要目的:以健康受试者为试验对象,采用开放、随机、两周期、双交叉试验设计,考察健康受试者空腹单次口服由江苏万禾制药有限公司生产(南京万融健诚医药科技有限公司持证)的苯磺酸美洛加巴林片(受试制剂,规格:5mg)与第一三共株式会社生产的苯磺酸美洛加巴林片(参比制剂, TARLIGE ®,规格:5mg)后美洛加巴林的体内经时过程,估算其相关药代动力学参数及相对生物利用度,评价其空腹状态下的生物等效性,为受试制剂的生产注册申请提供依据。
次要目的:评价苯磺酸美洛加巴林片受试制剂和参比制剂(商品名:TARLIGE®)在健康受试者中的安全性。
[Translation] Main purpose: Using healthy subjects as test subjects, an open, randomized, two-period, double-crossover design was used to investigate the in vivo time course of melogabalin after a single oral administration of melogabalin besylate tablets (test preparation, specification: 5 mg) produced by Jiangsu Wanhe Pharmaceutical Co., Ltd. (certified by Nanjing Wanrong Jiancheng Pharmaceutical Technology Co., Ltd.) and melogabalin besylate tablets (reference preparation, TARLIGE ®, specification: 5 mg) produced by Daiichi Sankyo Co., Ltd. on an empty stomach, to estimate their relevant pharmacokinetic parameters and relative bioavailability, to evaluate their bioequivalence under fasting conditions, and to provide a basis for the production registration application of the test preparation.
Secondary purpose: To evaluate the safety of the test preparation and reference preparation of melogabalin besylate tablets (trade name: TARLIGE®) in healthy subjects.
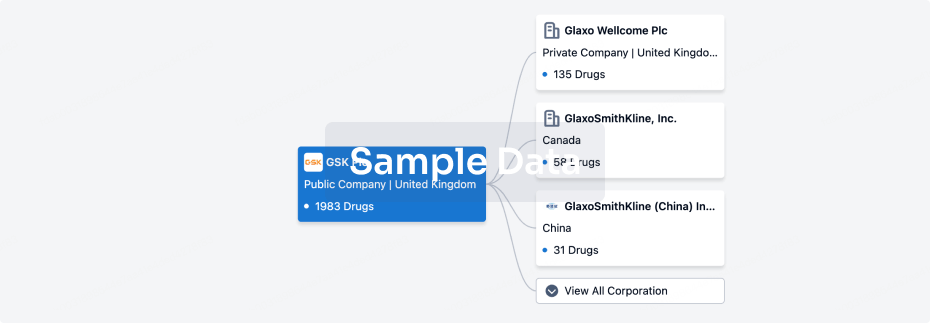
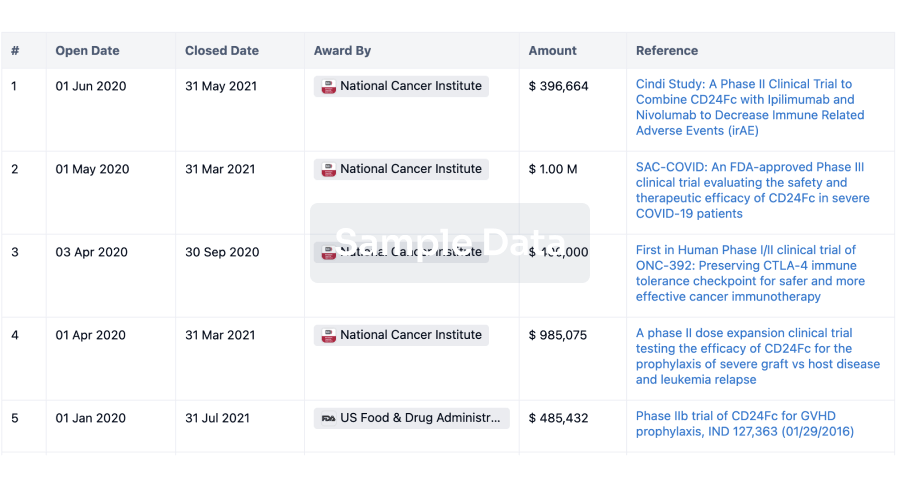
100 Clinical Results associated with Nanjing Wanrong Jiancheng Pharmaceutical Technology Co., Ltd.
0 Patents (Medical) associated with Nanjing Wanrong Jiancheng Pharmaceutical Technology Co., Ltd.
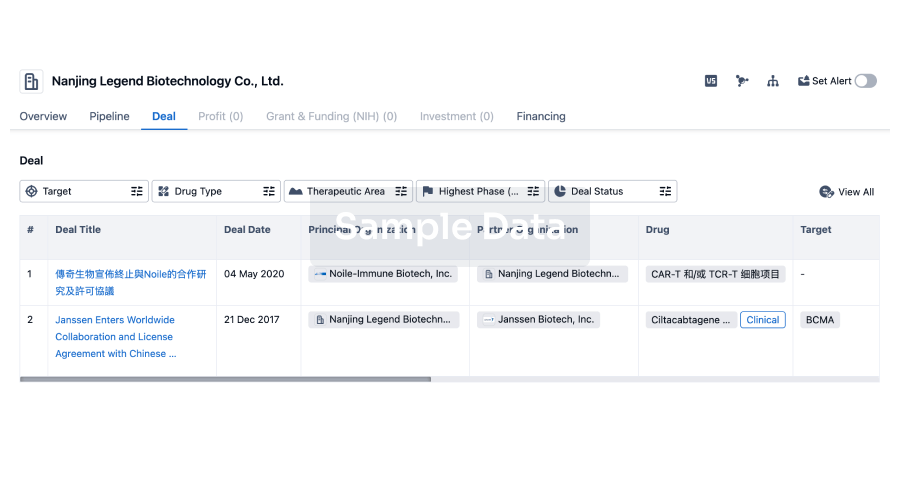
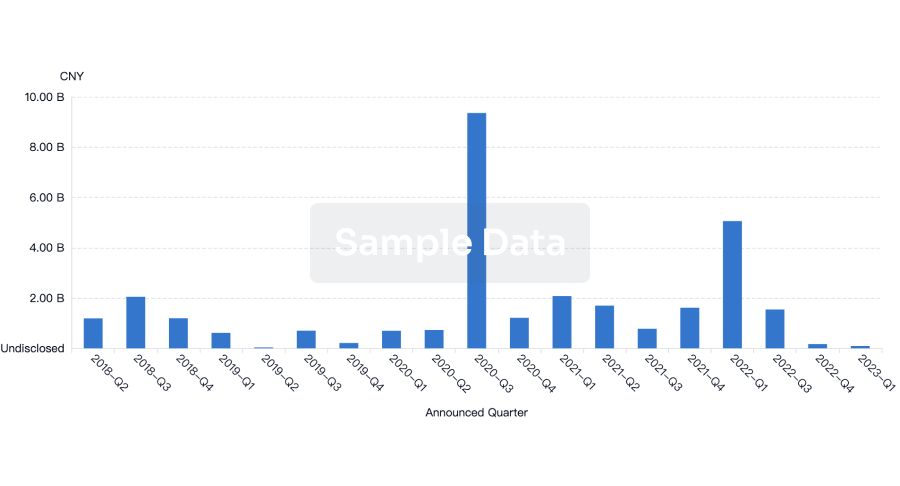
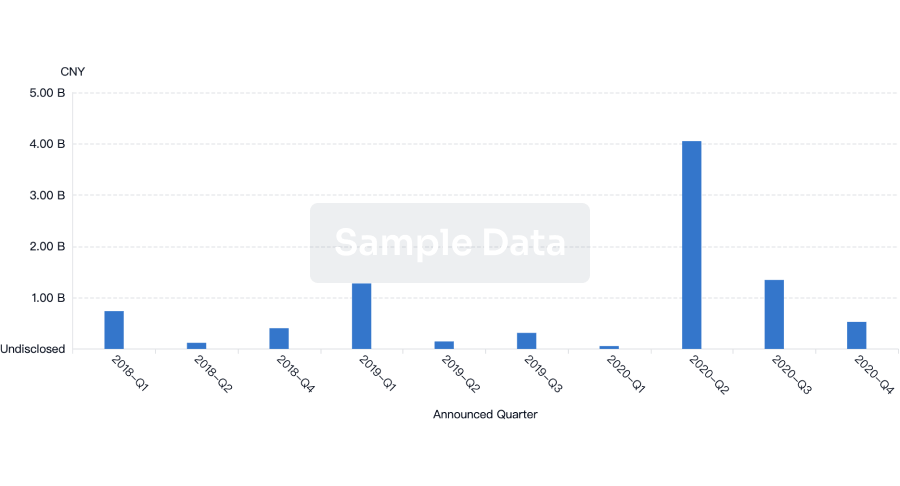
100 Deals associated with Nanjing Wanrong Jiancheng Pharmaceutical Technology Co., Ltd.
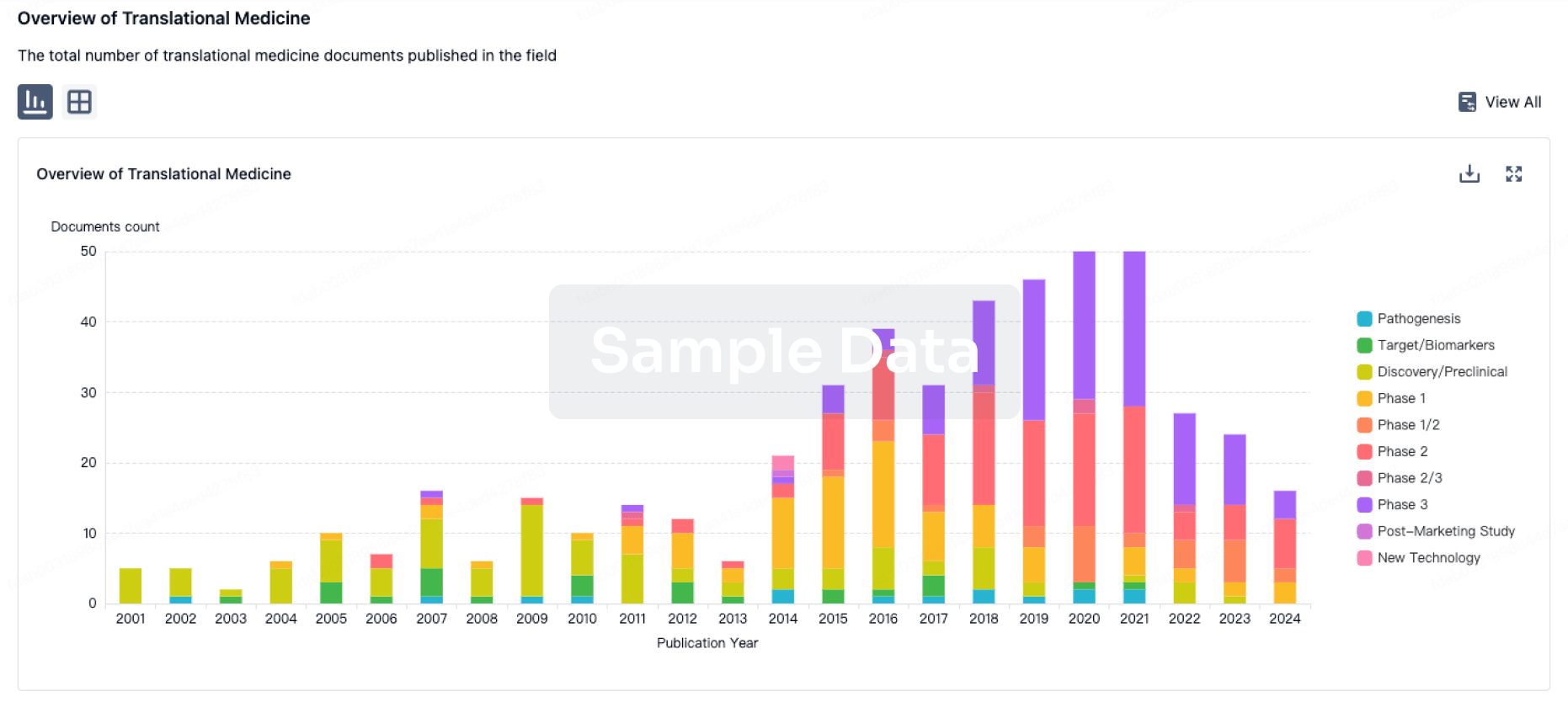
100 Translational Medicine associated with Nanjing Wanrong Jiancheng Pharmaceutical Technology Co., Ltd.