/ CompletedNot Applicable 氢溴酸替格列汀片在中国健康受试者中随机、开放、两制剂、单次给药、两周期、双交叉、空腹和餐后状态下的生物等效性试验
[Translation] A randomized, open-label, two-dose, single-dose, two-period, double-crossover, fasting and fed bioequivalence study of tenegliptin hydrobromide tablets in Chinese healthy subjects
主要研究目的:以广州汇元医药科技有限公司研发的氢溴酸替格列汀片(规格:20 mg)为受试制剂,田辺三菱製薬株式会社持证的氢溴酸替格列汀片(规格:20 mg,商品名:Tenelia®)为参比制剂,分别考察空腹和餐后状态下受试制剂与参比制剂在健康受试者体内的药代动力学参数,并评价两制剂的生物等效性。
次要研究目的:观察受试制剂和参比制剂在健康受试者中的安全性。
[Translation] Main research purpose: Take the texagliptin hydrobromide tablets (specification: 20 mg) developed by Guangzhou Huiyuan Pharmaceutical Technology Co., Ltd. as the test preparation, and the texagliptin hydrobromide tablets certified by Mitsubishi Tabe Co., Ltd. (Strength: 20 mg, trade name: Tenelia®) is a reference preparation. The pharmacokinetic parameters of the test preparation and the reference preparation in healthy subjects under fasting and postprandial conditions were respectively investigated, and the performance of the two preparations was evaluated. Bioequivalence.
Secondary research purpose: To observe the safety of test preparation and reference preparation in healthy subjects.
/ CompletedNot Applicable 非布司他片(40 mg)在健康受试者中随机、开放、两制剂、单次给药、两周期、双交叉、空腹和餐后状态下生物等效性试验
[Translation] A randomized, open-label, two-formulation, single-dose, two-period, double-crossover bioequivalence study of febuxostat tablets (40 mg) in healthy subjects under fasting and fed conditions
主要研究目的:以广州汇元医药科技有限公司持有的非布司他片(规格:40 mg)为受试制剂,TEIJIN PHARMA LIMITED IWAKUNI PHARMACEUTICAL FACTORY生产的非布司他片(规格:40 mg,商品名:菲布力®)为参比制剂,分别考察空腹和餐后状态下受试制剂与参比制剂在健康受试者体内的药代动力学参数,评价两制剂的生物等效性。
次要研究目的:观察受试制剂和参比制剂在健康受试者中的安全性。
[Translation] The main purpose of the study is to use the febuxostat tablets (specification: 40 mg) owned by Guangzhou Huiyuan Pharmaceutical Technology Co., Ltd. as the test preparation and the febuxostat tablets (specification: 40 mg, trade name: Feibuli®) produced by TEIJIN PHARMA LIMITED IWAKUNI PHARMACEUTICAL FACTORY as the reference preparation to investigate the pharmacokinetic parameters of the test preparation and the reference preparation in healthy subjects in the fasting and postprandial states, and evaluate the bioequivalence of the two preparations.
Secondary purpose of the study: to observe the safety of the test preparation and the reference preparation in healthy subjects.
/ CompletedNot Applicable 枸橼酸西地那非片(0.1 g)在健康男性受试者中随机、开放、两制剂、单次给药、空腹和餐后生物等效性研究
[Translation] A randomized, open-label, two-dose, single-dose, fasting and fed bioequivalence study of sildenafil citrate tablets (0.1 g) in healthy male subjects
主要目的:以广州汇元医药科技有限公司持有的枸橼酸西地那非片(0.1 g/片)为受试制剂,与辉瑞制药有限公司生产的枸橼酸西地那非片(100 mg/片)(商品名:万艾可®)为参比制剂,对比在健康人体内的吸收速度和吸收程度,考察两制剂的人体生物等效性。
次要目的:观察受试制剂和参比制剂在中国男性健康受试者中的安全性。
[Translation] Primary objective: To compare the absorption rate and extent of sildenafil citrate tablets (0.1 g/tablet) held by Guangzhou Huiyuan Pharmaceutical Technology Co., Ltd. (trade name: Viagra®) as the reference preparation, and to investigate the bioequivalence of the two preparations in healthy human bodies.
Secondary objective: To observe the safety of the test preparation and the reference preparation in healthy Chinese male subjects.
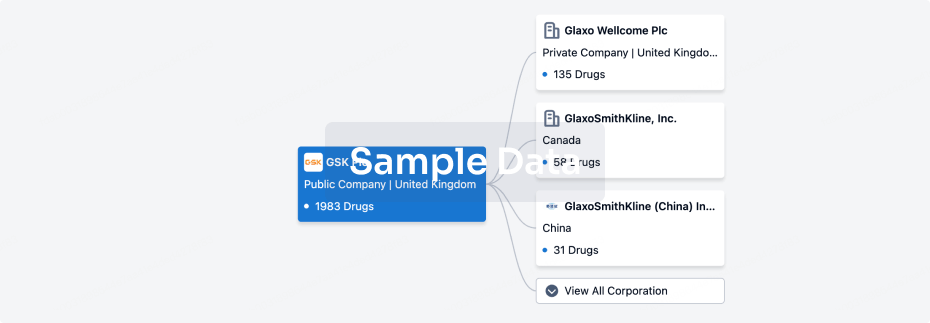
100 Clinical Results associated with Guangzhou Huiyuan Pharmaceutical Technology Co., Ltd.
0 Patents (Medical) associated with Guangzhou Huiyuan Pharmaceutical Technology Co., Ltd.
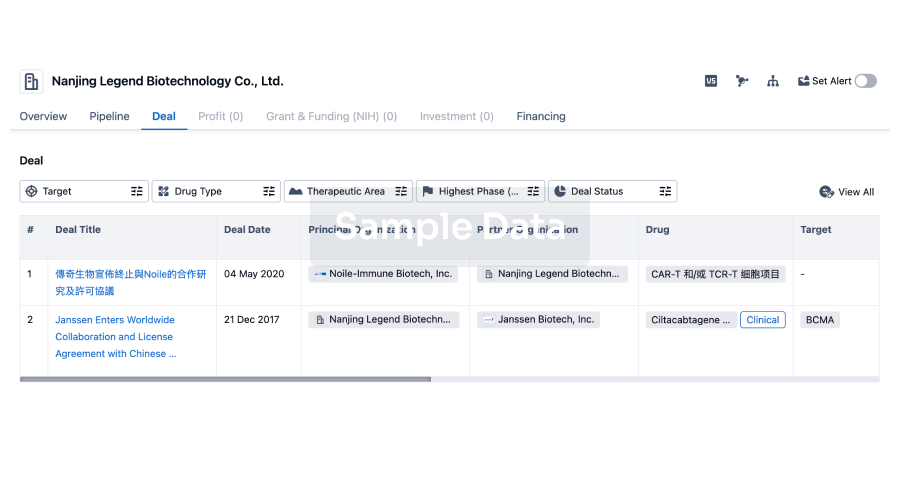
100 Deals associated with Guangzhou Huiyuan Pharmaceutical Technology Co., Ltd.
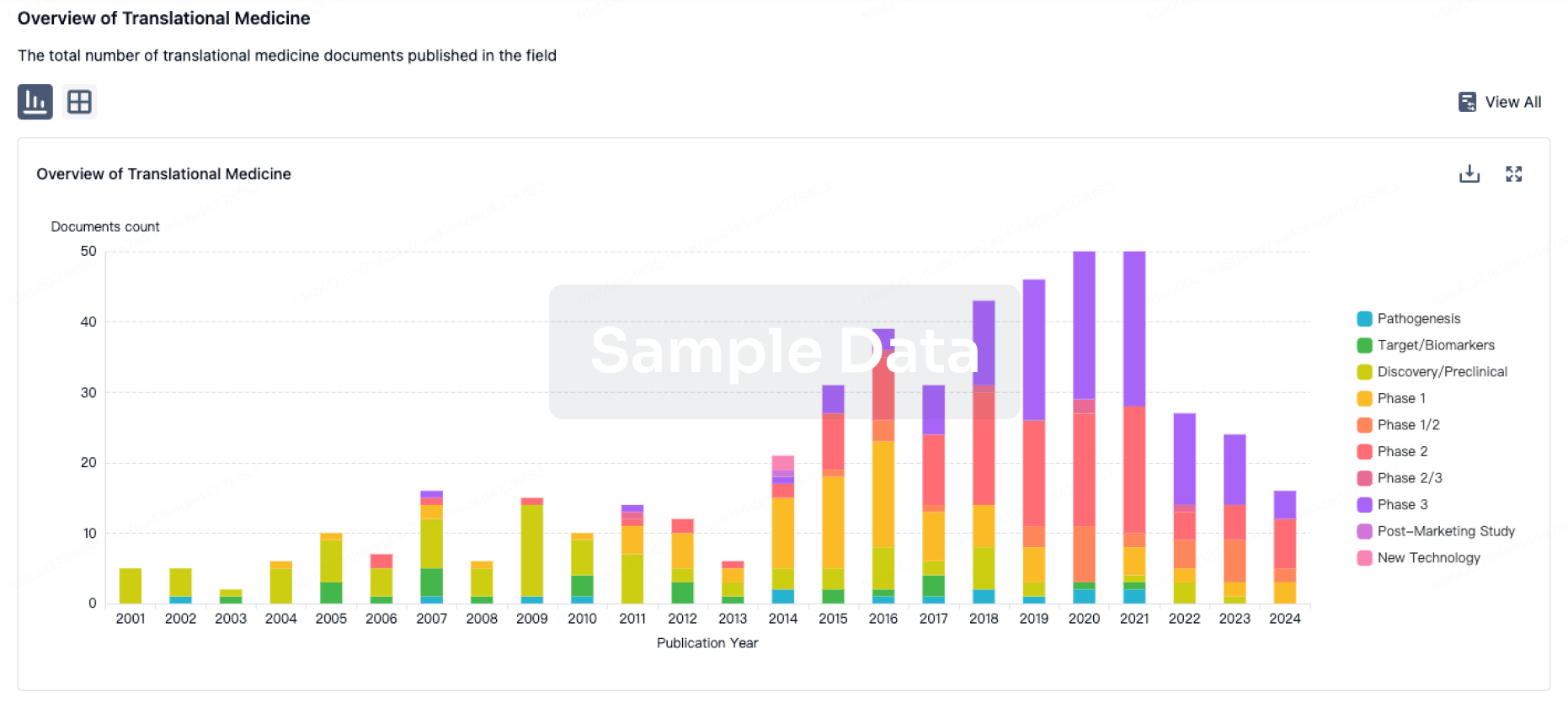
100 Translational Medicine associated with Guangzhou Huiyuan Pharmaceutical Technology Co., Ltd.