/ CompletedNot Applicable 匹维溴铵片在中国健康受试者中餐后状态下的单剂量、随机、开放、四周期、两序列、完全重复交叉的生物等效性正式试验
[Translation] A single-dose, randomized, open-label, four-period, two-sequence, fully repeated crossover bioequivalence study of pinaverium bromide tablets in Chinese healthy subjects in the fed state
主要研究目的
研究餐后状态下口服受试制剂(T)匹维溴铵片(规格:50 mg,华益药业科技(安徽)有限公司生产,广东稳健药业有限公司提供)与参比制剂(R)匹维溴铵片(商品名:Dicetel®,规格:50 mg,Abbott Laboratories Limited)在健康成年受试者体内的药代动力学,评价两种制剂的生物等效性。
次要研究目的
评价中国健康受试者餐后状态下口服受试制剂(T)匹维溴铵片和参比制剂(R)匹维溴铵片(Dicetel®)后的安全性。
[Translation] Main study objectives
To study the pharmacokinetics of the test preparation (T) Pinaverium Bromide Tablets (Specification: 50 mg, produced by Huayi Pharmaceutical Technology (Anhui) Co., Ltd., provided by Guangdong Wenjie Pharmaceutical Co., Ltd.) and the reference preparation (R) Pinaverium Bromide Tablets (trade name: Dicetel®, specification: 50 mg, Abbott Laboratories Limited) in healthy adult subjects after oral administration in the fed state, and to evaluate the bioequivalence of the two preparations.
Secondary study objectives
To evaluate the safety of the test preparation (T) Pinaverium Bromide Tablets and the reference preparation (R) Pinaverium Bromide Tablets (Dicetel®) after oral administration in healthy Chinese subjects in the fed state.
/ CompletedNot Applicable 中国健康受试者空腹状态下单次给予骨化三醇胶丸的随机、开放、两序列、两周期交叉设计生物等效性试验
[Translation] A randomized, open-label, two-sequence, two-period crossover bioequivalence study of a single dose of calcitriol softgels in Chinese healthy subjects under fasting conditions
主要目的:
研究空腹状态下单次口服骨化三醇胶丸(规格:0.25μg,广东稳健药业有限公司)与参比制剂(规格:0.25μg,罗盖全®;持证商:Roche Pharma (Schweiz)AG)后骨化三醇在中国健康受试者体内的药代动力学(PK)特征,评价空腹状态下口服两种制剂的生物等效性。
次要目的:
评价中国健康受试者在空腹状态下单次口服骨化三醇胶丸受试制剂和参比制剂后的安全性。
[Translation] Primary objective:
To study the pharmacokinetic (PK) characteristics of calcitriol in healthy Chinese subjects after a single oral administration of calcitriol capsules (specification: 0.25μg, Guangdong WenJian Pharmaceutical Co., Ltd.) and reference preparation (specification: 0.25μg, Rogaquan®; licensee: Roche Pharma (Schweiz) AG) in the fasting state, and to evaluate the bioequivalence of the two preparations in the fasting state.
Secondary objective:
To evaluate the safety of the test preparation and reference preparation of calcitriol capsules in healthy Chinese subjects after a single oral administration in the fasting state.
/ CompletedNot Applicable 中国健康受试者空腹和餐后状态下单次给予骨化三醇胶丸的随机、开放、两序列、两周期交叉设计生物等效性试验
[Translation] A randomized, open-label, two-sequence, two-period crossover bioequivalence study of a single dose of calcitriol softgels in Chinese healthy subjects under fasting and fed conditions
主要目的:
研究空腹和餐后状态下单次口服骨化三醇胶丸(规格:0.25μg,广东稳健药业有限公司)与参比制剂(规格:0.25μg,罗盖全®;持证商:Roche Pharma (Schweiz) Ltd.)后骨化三醇在中国健康受试者体内的药代动力学(PK)特征,评价空腹和餐后状态下口服两种制剂的生物等效性。
次要目的:
评价中国健康受试者在空腹和餐后状态下单次口服骨化三醇胶丸受试制剂和参比制剂后的安全性。
[Translation] Primary objective:
To study the pharmacokinetic (PK) characteristics of calcitriol in healthy Chinese subjects after a single oral administration of calcitriol capsules (specification: 0.25μg, Guangdong WenJian Pharmaceutical Co., Ltd.) and reference preparations (specification: 0.25μg, Rogaquan®; licensee: Roche Pharma (Schweiz) Ltd.) in fasting and fed states, and to evaluate the bioequivalence of the two preparations in fasting and fed states.
Secondary objective:
To evaluate the safety of the test preparation and reference preparation of calcitriol capsules in healthy Chinese subjects after a single oral administration in fasting and fed states.
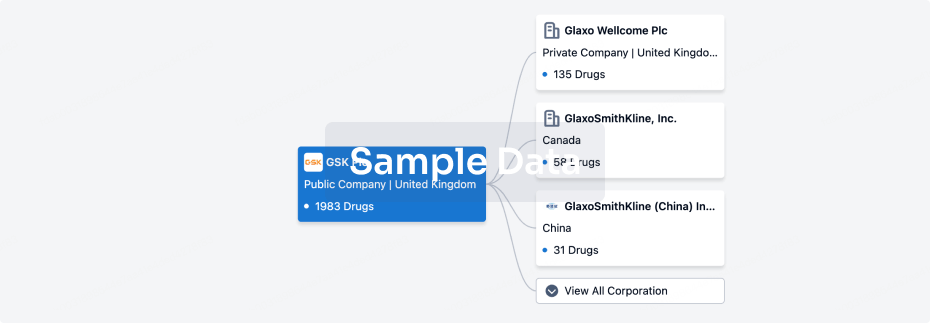
100 Clinical Results associated with Guangdong Wenjian Pharmaceutical Co.,Ltd.
0 Patents (Medical) associated with Guangdong Wenjian Pharmaceutical Co.,Ltd.
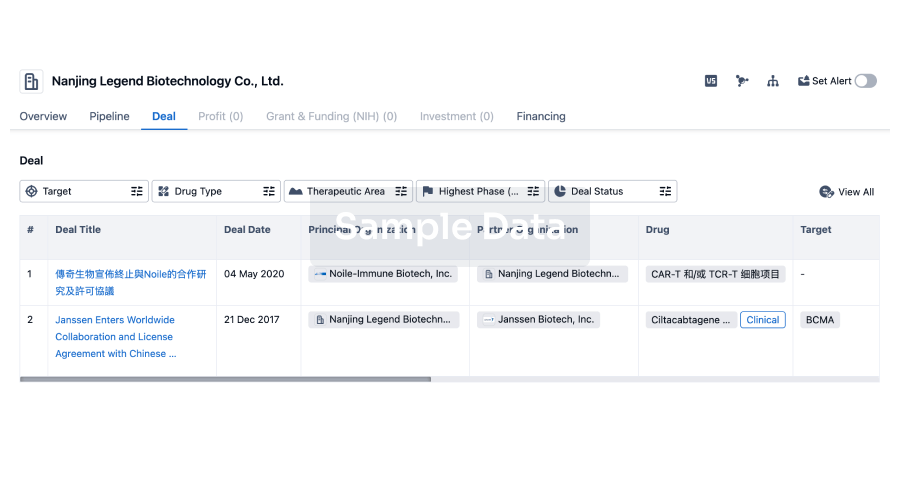
100 Deals associated with Guangdong Wenjian Pharmaceutical Co.,Ltd.
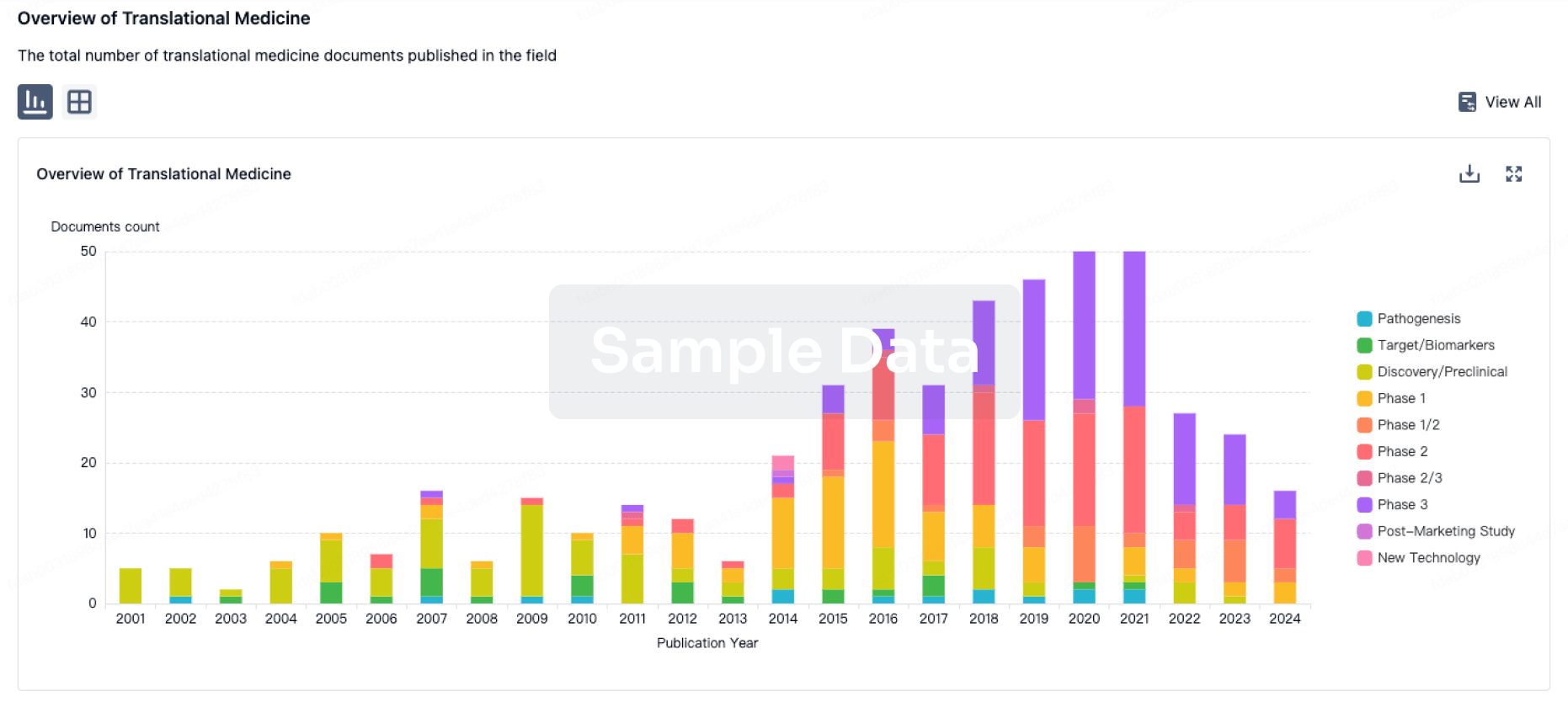
100 Translational Medicine associated with Guangdong Wenjian Pharmaceutical Co.,Ltd.