/ Not yet recruitingNot Applicable 多西他赛注射用浓缩液(自乳化型)在晚期乳腺癌或非小细胞肺癌患者的多中心、随机、开放、单剂量、两周期、双交叉的人体生物等效性试验
[Translation] A multicenter, randomized, open-label, single-dose, two-cycle, double-crossover bioequivalence study of docetaxel concentrate for injection (self-emulsifying type) in patients with advanced breast cancer or non-small cell lung cancer
主要目的
以Sanofi-Aventis Deutschland GmbH公司生产的多西他赛注射液(商品名:泰索帝®)(规格:0.5mL:20mg)为参比制剂,按照生物等效性试验的有关规定,与北京德立英捷医药科技有限公司研制的多西他赛注射用浓缩液(自乳化型)(规格:1mL:20mg)为受试制剂进行体内药动学对比研究,考察多西他赛在晚期乳腺癌或非小细胞肺癌患者体内的药代动力学行为,评价两种制剂的生物等效性。
次要目的
研究受试制剂多西他赛注射用浓缩液(自乳化型)和参比制剂多西他赛注射液(商品名:泰索帝®)在晚期乳腺癌或非小细胞肺癌患者中的安全性。
[Translation] Main purpose
The docetaxel injection (trade name: Taxotere®) (specification: 0.5mL: 20mg) produced by Sanofi-Aventis Deutschland GmbH was used as the reference preparation. According to the relevant provisions of the bioequivalence test, the docetaxel injection concentrate (self-emulsifying type) (specification: 1mL: 20mg) developed by Beijing Deli Yingjie Pharmaceutical Technology Co., Ltd. was used as the test preparation for in vivo pharmacokinetic comparison study to investigate the pharmacokinetic behavior of docetaxel in patients with advanced breast cancer or non-small cell lung cancer and evaluate the bioequivalence of the two preparations.
Secondary purpose
To study the safety of the test preparation docetaxel injection concentrate (self-emulsifying type) and the reference preparation docetaxel injection (trade name: Taxotere®) in patients with advanced breast cancer or non-small cell lung cancer.
多西他赛注射用浓缩液(自乳化型)的人体药代动力学对比研究
[Translation] Comparative study on the pharmacokinetics of docetaxel concentrate for injection (self-emulsifying type) in humans
主要目的:以北京德立英捷医药科技有限公司研制、生产的多西他赛注射用浓缩液(自乳化型)(规格:1mL:20mg)为受试制剂,以Sanofi-Aventis Deutschland GmbH公司生产的多西他赛注射液(规格:0.5mL:20mg,泰索帝®)为参比制剂,按照生物等效性试验的有关规定进行体内药动学对比研究,考察多西他赛在诊断为恶性肿瘤且准备接受多西他赛化疗方案患者中的药代动力学行为,评价两种制剂的生物等效性。
次要目的:研究受试制剂多西他赛注射用浓缩液(自乳化型)和参比制剂多西他赛注射液(商品名:泰索帝®)在诊断为恶性肿瘤且准备接受多西他赛化疗方案患者中的安全性
[Translation] Main purpose: To use docetaxel injection concentrate (self-emulsifying type) (specification: 1mL: 20mg) developed and produced by Beijing Deli Yingjie Pharmaceutical Technology Co., Ltd. as the test preparation, and docetaxel injection (specification: 0.5mL: 20mg, Taxotere®) produced by Sanofi-Aventis Deutschland GmbH as the reference preparation, and conduct an in vivo pharmacokinetic comparison study in accordance with the relevant provisions of the bioequivalence test to investigate the pharmacokinetic behavior of docetaxel in patients diagnosed with malignant tumors and ready to receive docetaxel chemotherapy, and evaluate the bioequivalence of the two preparations.
Secondary purpose: To study the safety of the test preparation docetaxel injection concentrate (self-emulsifying type) and the reference preparation docetaxel injection (trade name: Taxotere®) in patients diagnosed with malignant tumors and ready to receive docetaxel chemotherapy
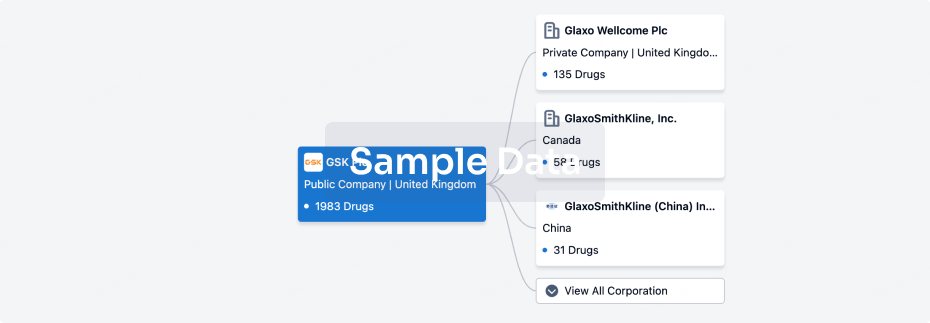
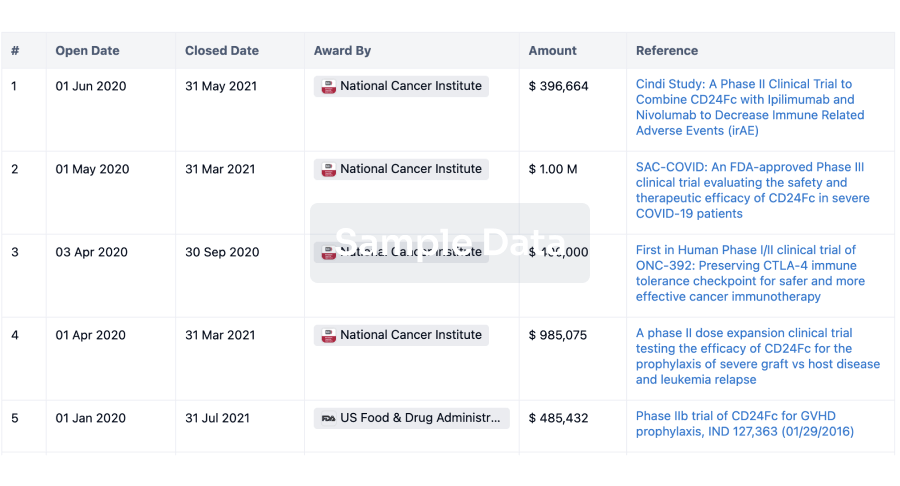
100 Clinical Results associated with Beijing Deli Yingjie Pharmaceutical Technology Co., Ltd.
0 Patents (Medical) associated with Beijing Deli Yingjie Pharmaceutical Technology Co., Ltd.
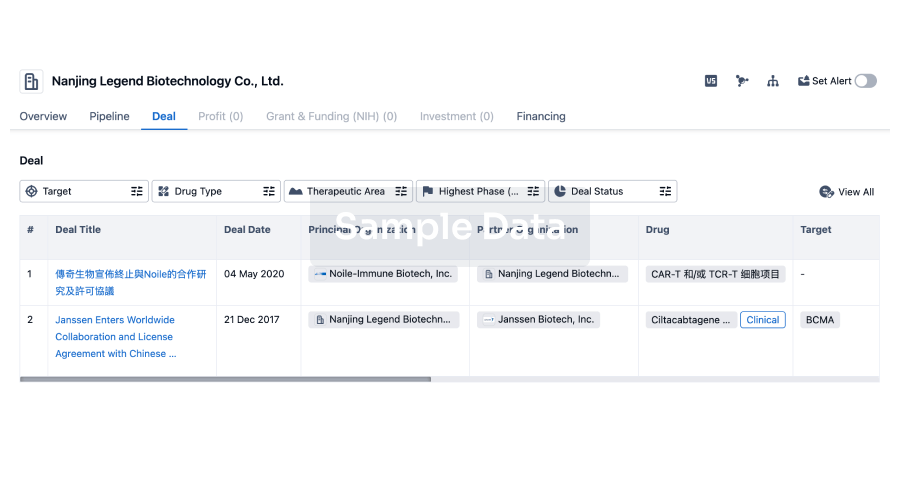
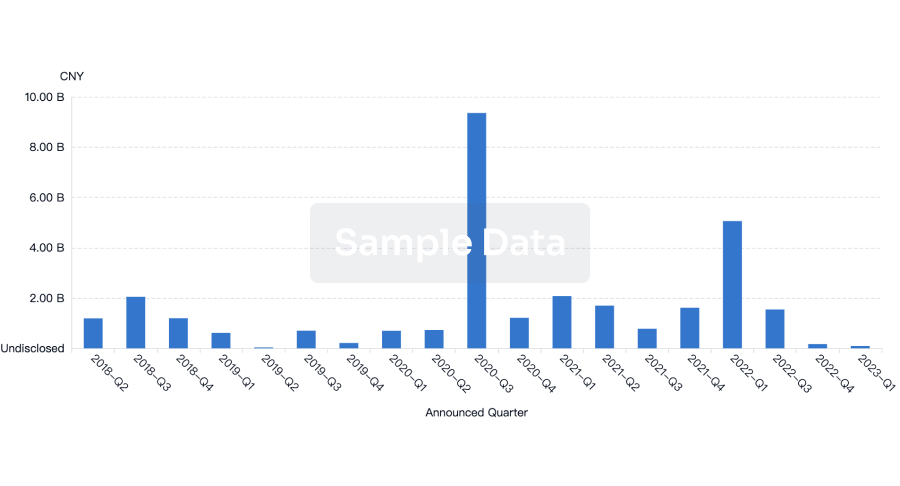
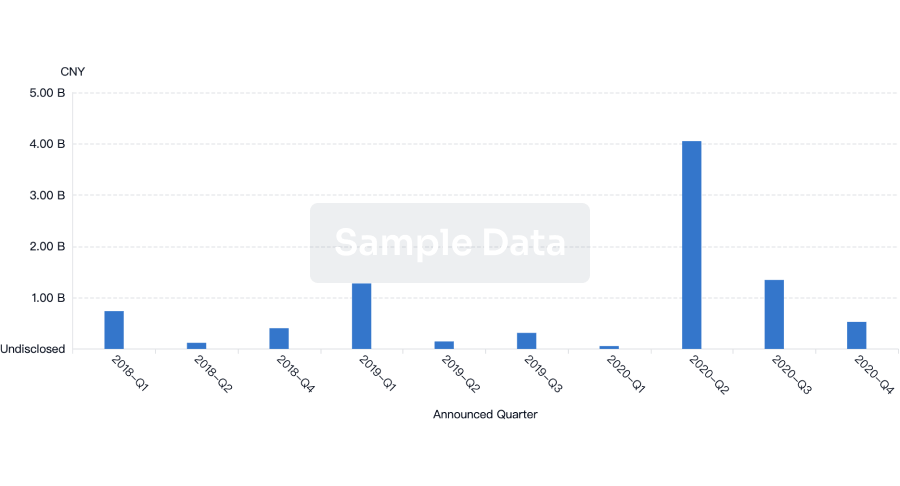
100 Deals associated with Beijing Deli Yingjie Pharmaceutical Technology Co., Ltd.
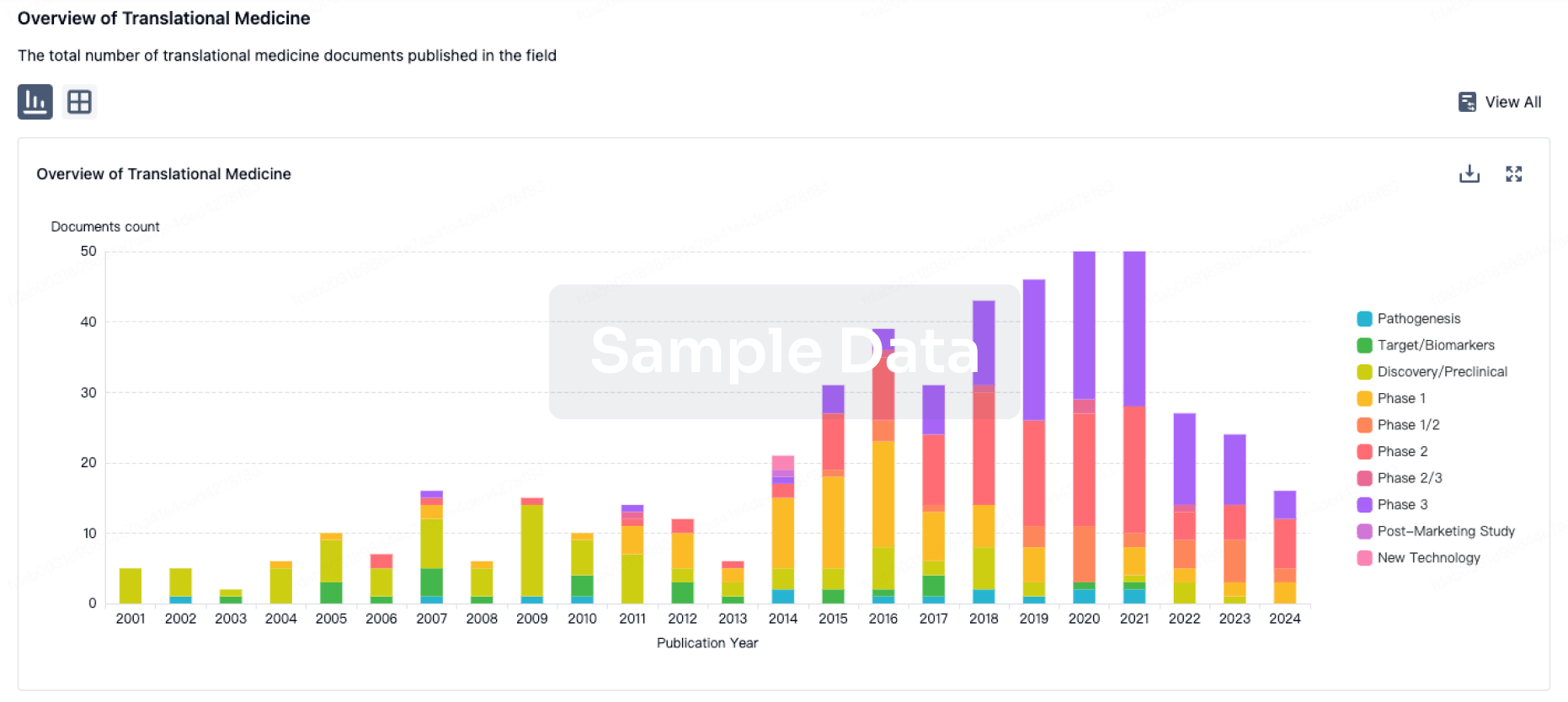
100 Translational Medicine associated with Beijing Deli Yingjie Pharmaceutical Technology Co., Ltd.