Request Demo
Last update 23 Jan 2025
RUNX2 x Alkaline phosphatase
Last update 23 Jan 2025
Basic Info
Related Targets |
Related
1
Drugs associated with RUNX2 x Alkaline phosphataseMechanism Alkaline phosphatase stimulants [+1] |
Active Org. |
Originator Org. |
Active Indication |
Inactive Indication- |
Drug Highest PhasePreclinical |
First Approval Ctry. / Loc.- |
First Approval Date20 Jan 1800 |
100 Clinical Results associated with RUNX2 x Alkaline phosphatase
Login to view more data
100 Translational Medicine associated with RUNX2 x Alkaline phosphatase
Login to view more data
0 Patents (Medical) associated with RUNX2 x Alkaline phosphatase
Login to view more data
1,630
Literatures (Medical) associated with RUNX2 x Alkaline phosphatase01 Dec 2025·Histochemistry and Cell Biology
Polycomb protein Bmi1 promotes odontoblast differentiation by accelerating Wnt and BMP signaling pathways
Article
Author: Ninomiya, Tadashi ; Kitamura, Chiaki ; Hosoya, Akihiro ; Seki-Kishimoto, Yuri ; Yukita, Akira ; Nakamura, Hiroaki ; Takebe, Hiroaki ; Iijima, Masahiro ; Noguchi, Yukiko ; Yoshiba, Nagako ; Washio, Ayako ; Morotomi, Takahiko
01 Feb 2025·Orthodontics & Craniofacial Research
Piezo1 Promotes Osteogenesis Through C aMKII Signalling in a Rat Maxillary Expansion Model
Article
Author: Yang, Kai ; Du, Yugui ; Tang, Jiayue ; Xu, Bowen ; Peng, Chuhan ; He, Zhengquan ; Liu, Runxuan
01 Feb 2025·Biotechnology Letters
Butea monosperma bark extract: a natural boost for osteogenesis via Wnt/β-catenin pathway activation in adipose-derived mesenchymal stem cells
Article
Author: Abraham, Annie ; John, Annie ; Sundar, Rebu ; Sundar, Gayathri
Analysis
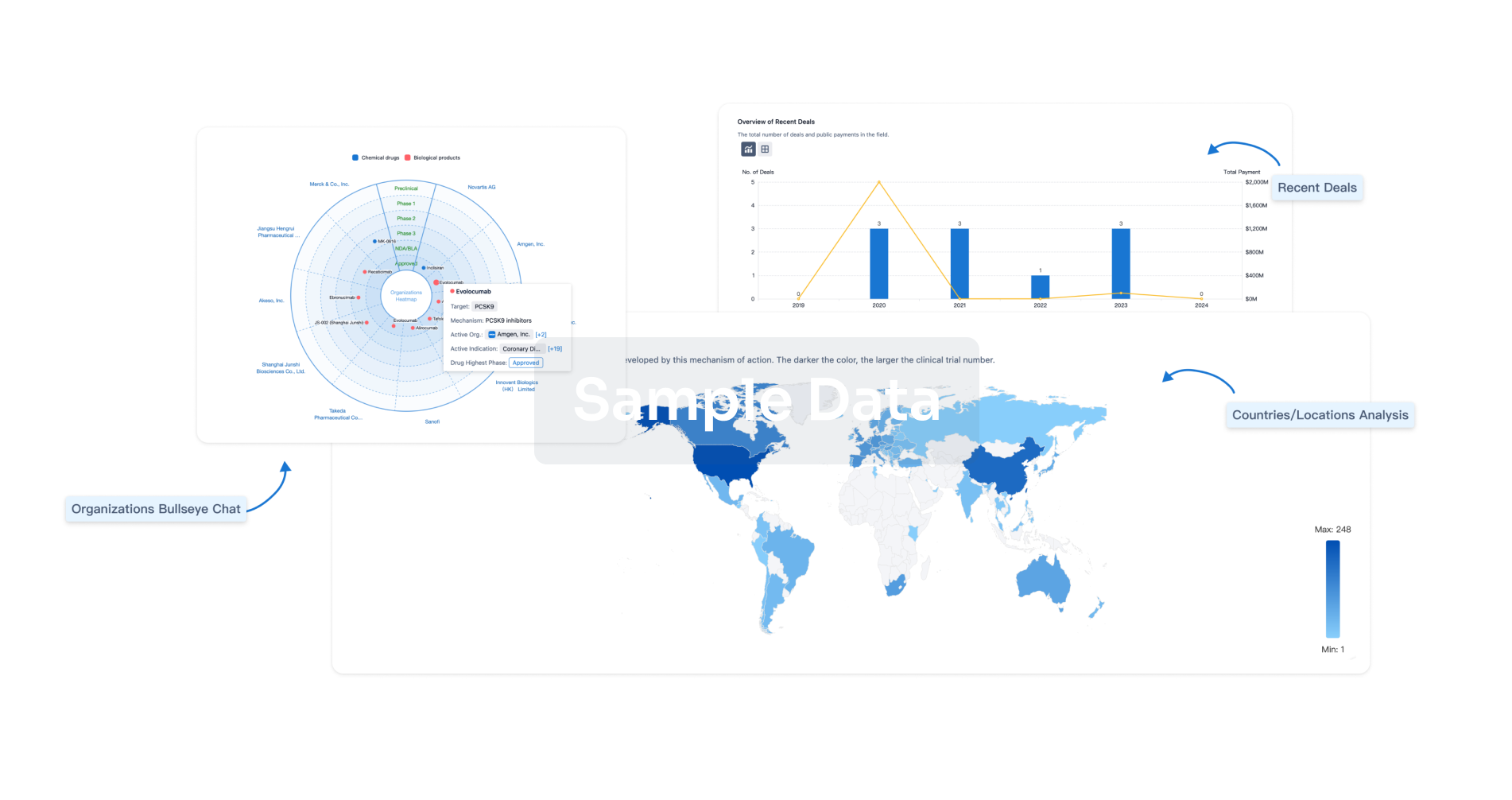
Perform a panoramic analysis of this field.
login
or
Chat with Hiro
Get started for free today!
Accelerate Strategic R&D decision making with Synapse, PatSnap’s AI-powered Connected Innovation Intelligence Platform Built for Life Sciences Professionals.
Start your data trial now!
Synapse data is also accessible to external entities via APIs or data packages. Empower better decisions with the latest in pharmaceutical intelligence.
Bio
Bio Sequences Search & Analysis
Sign up for free
Chemical
Chemical Structures Search & Analysis
Sign up for free
