Request Demo
Last update 03 Oct 2024
GBA1
Last update 03 Oct 2024
Basic Info
Related Targets |
Related
39
Drugs associated with GBA1Target |
Mechanism GBA stimulants |
Active Org. |
Originator Org. |
Active Indication |
Inactive Indication- |
Drug Highest PhaseApproved |
First Approval Ctry. / Loc. United States |
First Approval Date01 May 2012 |
Target |
Mechanism GBA stimulants |
Originator Org. |
Active Indication |
Inactive Indication- |
Drug Highest PhaseApproved |
First Approval Ctry. / Loc. United States |
First Approval Date23 May 1994 |
Target |
Mechanism GBA modulators [+1] |
Active Org. |
Originator Org. |
Active Indication |
Inactive Indication- |
Drug Highest PhasePhase 2 |
First Approval Ctry. / Loc.- |
First Approval Date- |
46
Clinical Trials associated with GBA1CTIS2024-511172-33-00
A Multicenter, Long-term, Follow-up Study to Investigate the Safety and Durability of Response Following Dosing of an Adeno-associated Viral Vector (FLT201) in Subjects with Gaucher Disease (GALILEO-2) - FLT201-02
Start Date13 May 2024 |
Sponsor / Collaborator |
NCT06545136
A Multicenter, Long-term, Follow-up Study to Investigate the Safety and Durability of Response Following Dosing of an Adeno-associated Viral Vector (FLT201) in Subjects With Gaucher Disease (GALILEO-2)
This is a multicenter, long-term, follow-up trial of participants with Gaucher disease type 1 who received FLT201 treatment in a preceding clinical trial. Participants will be followed for 5 years post-treatment.
Start Date13 May 2024 |
Sponsor / Collaborator |
NCT06162338
A Prospective, Single-center, Open-arm, Single-arm Study of the Safety and Preliminary Efficacy of Single Intravenous Infusion Administration of LY-M001 Injection in the Treatment of Adult Patients With Gaucher Disease Type I
This is a prospective single-center, open, single-arm, single-dose intravenous infusion study to evaluate the safety and initial efficacy, pharmacodynamic characteristics, immunogenicity, biodistribution, and viral shedding of LY-M001 injection.This study mainly includes the main study stage and the long-term follow-up study stage.
Start Date30 Oct 2023 |
Sponsor / Collaborator  Zhejiang University Zhejiang University [+1] |
100 Clinical Results associated with GBA1
Login to view more data
100 Translational Medicine associated with GBA1
Login to view more data
0 Patents (Medical) associated with GBA1
Login to view more data
3,638
Literatures (Medical) associated with GBA101 Dec 2024·CELLULAR AND MOLECULAR LIFE SCIENCES
Lysosomal dysfunction in α-synuclein pathology: molecular mechanisms and therapeutic strategies
Review
Author: Dai, Lijun ; Zhang, Zhentao ; Chen, Liam ; Fang, Xin ; Ke, Wei ; Liu, Miao
In orchestrating cell signaling, facilitating plasma membrane repair, supervising protein secretion, managing waste elimination, and regulating energy consumption, lysosomes are indispensable guardians that play a crucial role in preserving intracellular homeostasis. Neurons are terminally differentiated post-mitotic cells. Neuronal function and waste elimination depend on normal lysosomal function. Converging data suggest that lysosomal dysfunction is a critical event in the etiology of Parkinson's disease (PD). Mutations in Glucosylceramidase Beta 1 (GBA1) and leucine-rich repeat kinase 2 (LRRK2) confer an increased risk for the development of parkinsonism. Furthermore, lysosomal dysfunction has been observed in the affected neurons of sporadic PD (sPD) patients. Given that lysosomal hydrolases actively contribute to the breakdown of impaired organelles and misfolded proteins, any compromise in lysosomal integrity could incite abnormal accumulation of proteins, including α-synuclein, the major component of Lewy bodies in PD. Clinical observations have shown that lysosomal protein levels in cerebrospinal fluid may serve as potential biomarkers for PD diagnosis and as signs of lysosomal dysfunction. In this review, we summarize the current evidence regarding lysosomal dysfunction in PD and discuss the intimate relationship between lysosomal dysfunction and pathological α-synuclein. In addition, we discuss therapeutic strategies that target lysosomes to treat PD.
01 Nov 2024·BIOCHEMICAL AND BIOPHYSICAL RESEARCH COMMUNICATIONS
Upregulation of NFE2L1 reduces ROS levels and α-synuclein aggregation caused by GBA1 knockdown
Article
Author: Jiang, Nan ; Li, Yajun ; Shen, Fei ; Ma, Duan ; Wen, Shuxia ; Xiang, Wanqing ; Zhang, Jin
Biallelic mutations in the GBA1 gene result in Gaucher disease (GD), and both patients with GD and carriers of a single GBA1 mutation have an increased susceptibility to Parkinson's disease (PD), but the underlying mechanisms of this association are not yet clear. In previous studies, we established Gba1 F213I point mutation mice and found that homozygous Gba1 F213I mutant mice died shortly after birth, while heterozygous mice could survive normally. In this study, we investigated the transcriptomic changes in the brain tissue of Gba1 F213I heterozygous mice, identifying 138 differentially expressed genes. Among them, Nfe2l1 was the most significantly downregulated gene. Inhibition or knockdown of GBA1 in BE(2)-M17 cells resulted in decreased expression levels of NFE2L1. Knockdown of GBA1 or NFE2L1 could lead to an elevation in intracellular aggregation of α-synuclein (α-syn) and reactive oxygen species (ROS) levels, while upregulation of NFE2L1 effectively mitigated those cellular manifestations induced by GBA1 knockdown. In summary, our in vitro results showed that upregulation of NFE2L1 may provide a therapeutic benefit for cellular phenotypes resulting from GBA1 knockdown, providing new insights for future research on GD and GBA1-associated PD.
01 Nov 2024·JOURNAL OF COMPARATIVE PATHOLOGY
Striking and widespread microglial activation in the brains of Southdown lambs with type II glucocerebrosidosis (neuronopathic Gaucher disease)
Article
Author: Brealey, John ; Robertson, Thomas ; Finnie, John ; Hemsley, Kim ; Blumbergs, Peter ; Manavis, Jim ; Beard, Helen
Glucocerebrosidosis (termed Gaucher disease in humans) is a lysosomal storage disease, caused by a deficiency of the enzyme glucocerebrosidase, which results in accumulation of the glycolipid substrate glucocerebroside in the macrophage-monocyte system. Three principal forms are recognized in humans, two being neuronopathic and resulting in neurodegeneration. Only two spontaneously arising cases have been described in domestic animals, one in a dog and the other in a flock of Southdown sheep. Since microglial activation is increasingly being recognized as having an important role in the pathogenesis of Gaucher disease and archival brains were available from lambs with type II glucocerebrosidosis, we wanted to determine whether microglia were activated in these brains. Ionized calcium binding adaptor molecule 1 (Iba1), a specific and the most widely expressed immunohistochemical marker of microglial activation, was used. Striking and widely distributed activation of microglia was demonstrated, suggesting that microglia actively participate in the development of neuropathological changes in ovine Gaucher disease. This aspect of Gaucher disease requires further study in any future cases detected in domestic animal species, including the mechanism by which this markedly increased Iba1 expression is related to disease progression.
175
News (Medical) associated with GBA130 Sep 2024
GT-02287 Increases Peripheral GCase Activity GT-02287 Demonstrates CNS Exposure Company to Host Webinar to Discuss Results at 8:30 AM ET Today BETHESDA, Md., Sept. 30, 2024 (GLOBE NEWSWIRE) -- Gain Therapeutics, Inc. (Nasdaq: GANX) (“Gain”, or the “Company”), a clinical-stage biotechnology company leading the discovery and development of the next generation of allosteric small molecule therapies, today announced the presentation of a late-breaking abstract at the International Congress of Parkinson’s Disease and Movement Disorders. The poster and oral platform presentation summarize the safety, tolerability, pharmacokinetics (PK), and pharmacodynamics of single- and multiple-ascending doses of GT-02287, the Company’s clinical stage lead drug candidate, in a Phase 1 first-in-human study in healthy volunteers. The International Congress of Parkinson’s Disease and Movement Disorders® is being held September 27 - October 1 in Philadelphia, PA. “The fact that we saw an increase in GCase activity in healthy volunteers, who we assume have homeostatic mechanisms to counter-regulate a pharmacologically-induced increase in GCase enzymatic activity, indicates that it will have more pronounced effect on GCase in people with Parkinson’s disease (PD) and further confirms our conviction that GT-02287 may be able to slow or stop the progression of PD. We hope that one day we can deliver this drug to those that need it and help them improve their everyday quality of life,” commented Jonas Hannestad, M.D., Ph.D., Chief Medical Officer of Gain. Gene Mack, interim Chief Executive Officer and current Chief Financial Officer of Gain, continued, “We remain enthusiastic about the promising profile of GT-02287 and look forward to initiating a trial in people with PD by the end of Q4 2024, with the goal of demonstrating safety and tolerability in patients with PD and obtaining proof of mechanism based on relevant biomarkers.” The late-breaker, titled, “The novel glucocerebrosidase chaperone GT-02287 in development for GBA-PD is safe and well tolerated in healthy volunteers at oral doses that produce plasma exposures in the projected therapeutic range,” was presented on-site by Jonas Hannestad and included results from the Phase 1 study of GT-02287. The single and multiple dose levels tested were safe and generally well tolerated, with no serious adverse events or Grade 3 (severe) adverse events observed, and no other safety signals detected. The PK profile of GT-02287 was linear across the tested dose ranges, and plasma exposures at daily doses of 7.7 mg/kg and above were within the projected therapeutic range. GT-02287 was measurable in cerebrospinal fluid (CSF) at levels in line with rodent levels at effective doses, demonstrating CNS exposure. Notably, GCase activity in dried blood spots increased approximately 30% in subjects who received GT-02287 but not in those who received placebo, demonstrating target engagement and modulation of GCase enzyme. GCase activity continued to increase 12 hours post-dose at 14 days, the furthest time point analyzed in the study. These results support the continued development of GT-02287 and its potential as a disease-modify treatment for Parkinson’s disease. A PDF of the poster presented at the International Congress of Parkinson’s Disease and Movement Disorders is available on the Science and Technology section of the Company’s website at https://gaintherapeutics.com/science-and-technology/posters. Webinar Details Gain Therapeutics will host a webinar on Monday, September 30 at 8:30 AM ET to review results presented at the International Congress of Parkinson’s Disease and Movement Disorders. To register, please click here: https://lifescievents.com/event/gaintherapeutics/ About GT-02287Gain Therapeutics’ lead drug candidate, GT-02287, is in clinical development for the treatment of Parkinson’s disease with or without a GBA1 mutation. The orally administered, brain-penetrant small molecule is an allosteric protein modulator that restores the function of the lysosomal protein enzyme glucocerebrosidase (GCase) which becomes misfolded and impaired due to mutations in the GBA1 gene, the most common genetic abnormality associated with PD, or other age-related stress factors. In preclinical models of PD, GT-02287 restored GCase enzymatic function, reduced aggregated α-synuclein, neuroinflammation and neuronal death, and improved motor function and cognitive performance. Additionally, GT-02287 significantly reduced plasma neurofilament light chain (NfL) levels, an emerging biomarker for neurodegeneration. Compelling preclinical data in mouse models of GBA1-PD, including that presented at FENS Forum 2024 in June describing improvement in cognitive performance in addition to motor performance after administration of GT-02287, suggests that GT-02287 may have the potential to slow the progression of Parkinson’s disease. Gain’s lead program in Parkinson’s disease has been awarded funding support from The Michael J. Fox Foundation for Parkinson’s Research (MJFF) and The Silverstein Foundation for Parkinson’s with GBA, as well as from the Eurostars-2 joint program with co-funding from the European Union Horizon 2020 research and Innosuisse – Swiss Innovation Agency. About Gain Therapeutics, Inc.Gain Therapeutics, Inc. is a clinical-stage biotechnology company leading the discovery and development of next generation allosteric therapies. Gain’s lead drug candidate, GT-02287 for the treatment of Parkinson’s disease with or without a GBA1 mutation, is currently being evaluated in a Phase 1 clinical trial. Leveraging AI-supported structural biology, proprietary algorithms, and supercomputer-powered physics-based models, the company’s Magellan™ drug discovery platform can identify novel allosteric binding sites on disease-implicated proteins, pinpointing pockets that cannot be found or drugged with current technologies. Its AI and machine-learning tools and virtual screening capabilities leverage the emerging on-demand compound libraries covering vast chemical spaces of over five trillion compounds to identify and select suitable small molecule hits for experimental validation. Gain’s unique approach enables the discovery of novel, allosteric small molecule modulators that can restore or disrupt protein function. Deploying its highly advanced platform, Gain is accelerating drug discovery and unlocking novel disease-modifying treatments for untreatable or difficult-to-treat disorders including neurodegenerative diseases, rare genetic disorders and oncology. Forward-Looking StatementsThis release contains “forward-looking statements” made pursuant to the safe harbor provisions of the Private Securities Litigation Reform Act of 1995. These statements are typically preceded by words such as “believes,” “expects,” “anticipates,” “intends,” “will,” “may,” “should,” or similar expressions. These forward-looking statements reflect management’s current knowledge, assumptions, judgment and expectations regarding future performance or events. Although management believes that the expectations reflected in such statements are reasonable, they give no assurance that such expectations will prove to be correct or that those goals will be achieved, and you should be aware that actual results could differ materially from those contained in the forward-looking statements. Forward-looking statements are subject to a number of risks and uncertainties, including, but not limited to, risks associated with market conditions and the satisfaction of customary closing conditions related to the offering and uncertainties related to the offerings and the use of proceeds from the offerings. For a further description of the risks and uncertainties that could cause actual results to differ from those expressed in these forward-looking statements, as well as risks relating to the Company’s business in general, please refer to the Company’s prospectus supplement to be filed with the SEC, and the documents incorporated by reference therein, including the Company’s Form 10-K for the year ended December 31, 2023 and Form 10-Q for the quarter ended June 30, 2024. All forward-looking statements are expressly qualified in their entirety by this cautionary notice. You are cautioned not to place undue reliance on any forward-looking statements, which speak only as of the date of this release. We have no obligation, and expressly disclaim any obligation, to update, revise or correct any of the forward-looking statements, whether as a result of new information, future events or otherwise. Investor Contacts:Apaar Jammu and Chuck Padalaajammu@gaintherapeutics.comchuck@lifesciadvisors.com Media Contacts:Russo PartnersNic Johnson and Elio Ambrosionic.johnson@russopartnersllc.comelio.ambrosio@russopartnersllc.com(760) 846-9256
Phase 1Clinical Result
27 Sep 2024
- Congruence's Revenir™ drug discovery engine identified diverse correctors of GCase designed to target the key biological dysfunction in Parkinson's disease patients with GBA1 mutations -
- Congruence is optimizing several GCase-targeting compounds for clinical development -
MONTREAL, Sept. 27, 2024 /PRNewswire/ -- Congruence Therapeutics, a computationally-driven biotechnology company building a unique pipeline of transformative small molecule correctors rationally designed to rescue aberrant protein function, announced today a poster presentation at the International Congress of Parkinson's Disease and Movement Disorders 2024 Meeting, being held September 27-October 1, 2024, in Philadelphia, PA.
"Mutations of the GBA1 gene, encoding the enzyme GCase, represents the most important genetic risk factor for Parkinson's Disease. At Congruence, we are developing small molecules designed to correct this biological deficit and our efforts have yielded promising preclinical results," said Sharath Hegde, Ph.D., Chief Scientific Officer of Congruence. "We look forward to presenting data demonstrating the use of our proprietary platform Revenir™ to discover potent and brain-penetrant allosteric GCase correctors which augment wild-type and mutant GCase activity in robust cellular assays. Optimization of these chemical leads is ongoing to discover potential clinical candidates."
Poster presentation:
Title: "Identification of Allosteric GCase Correctors using Revenir™ for the treatment of GBA1-Parkinson's Disease"
Abstract Number: 791
Session Date and Time: Sunday, September 29th, 1-3PM
Presenting Author: Jeremy Dupaul-Chicoine, Associate Director, Biology, Congruence Therapeutics
About Congruence Therapeutics
Congruence is a computationally-driven biotechnology company building a unique pipeline of transformative small molecule correctors rationally designed to rescue aberrant protein function. Our proprietary scalable platform, Revenir™, captures the biophysical features of proteins across their conformational ensembles, in order to identify novel allosteric and cryptic pockets which are virtually screened to generate novel chemical matter.
For more information, please visit
.
Company Contact
Charles Grubsztajn
Chief Operating Officer
[email protected]
Media Contact
Amy Conrad
Juniper Point
[email protected]
858-366-3243
SOURCE Congruence Therapeutics
WANT YOUR COMPANY'S NEWS FEATURED ON PRNEWSWIRE.COM?
440k+
Newsrooms &
Influencers
9k+
Digital Media
Outlets
270k+
Journalists
Opted In
GET STARTED

Clinical Study
26 Sep 2024
Company To Discuss Positive Results Presented at the International MDS Congress and the Design of Phase 1b Trial in Parkinson’s Disease Patients Event Will Be Held on Monday, September 30, 2024, at 8:30 am ET BETHESDA, Md., Sept. 26, 2024 (GLOBE NEWSWIRE) -- Gain Therapeutics, Inc. (Nasdaq: GANX) (“Gain”, or the “Company”), a clinical-stage biotechnology company leading the discovery and development of the next generation of allosteric small molecule therapies, today announced it is holding a webinar to discuss data from the Phase 1 study of GT-02287, a novel GCase-targeting small molecule therapy for Parkinson’s disease, recently presented in a late-breaker at the International Congress of Parkinson’s Disease and Movement Disorders® (MDS). The Company will also discuss the design of a planned Phase 1b trial of GT-02287 in Parkinson’s disease patients. Webinar Details Date: Monday, September 30, 2024 Time: 8:30 am ET Register for the event HERE or join the conference call through the News and Events section of the Company website at https://www.gaintherapeutics.com/investors-media/news-events. A live question and answer session will follow the formal presentations. A replay of the call will be available in the News and Events section of the Company website after the event. About GT-02287Gain Therapeutics’ lead drug candidate, GT-02287, is in clinical development for the treatment of Parkinson’s disease with or without a GBA1 mutation. The orally administered, brain-penetrant small molecule is an allosteric protein modulator that restores the function of the lysosomal protein enzyme glucocerebrosidase (GCase) which becomes misfolded and impaired due to mutations in the GBA1 gene, the most common genetic abnormality associated with PD, or other age-related stress factors. In preclinical models of PD, GT-02287 restored GCase enzymatic function, reduced aggregated α-synuclein, neuroinflammation and neuronal death, and improved motor function and cognitive performance. Additionally, GT-02287 significantly reduced plasma neurofilament light chain (NfL) levels, an emerging biomarker for neurodegeneration. Compelling preclinical data in mouse models of GBA1-PD, including that presented at FENS Forum 2024 in June describing improvement in cognitive performance in addition to motor performance after administration of GT-02287, suggests that GT-02287 may have the potential to slow the progression of Parkinson’s disease. Gain’s lead program in Parkinson’s disease has been awarded funding support from The Michael J. Fox Foundation for Parkinson’s Research (MJFF) and The Silverstein Foundation for Parkinson’s with GBA, as well as from the Eurostars-2 joint program with co-funding from the European Union Horizon 2020 research and Innosuisse – Swiss Innovation Agency. About Gain Therapeutics, Inc.Gain Therapeutics, Inc. is a clinical-stage biotechnology company leading the discovery and development of next generation allosteric therapies. Gain’s lead drug candidate, GT-02287 for the treatment of Parkinson’s disease with or without a GBA1 mutation, is currently being evaluated in a Phase 1 clinical trial. Leveraging AI-supported structural biology, proprietary algorithms, and supercomputer-powered physics-based models, the company’s Magellan™ drug discovery platform can identify novel allosteric binding sites on disease-implicated proteins, pinpointing pockets that cannot be found or drugged with current technologies. Its AI and machine-learning tools and virtual screening capabilities leverage the emerging on-demand compound libraries covering vast chemical spaces of over five trillion compounds to identify and select suitable small molecule hits for experimental validation. Gain’s unique approach enables the discovery of novel, allosteric small molecule modulators that can restore or disrupt protein function. Deploying its highly advanced platform, Gain is accelerating drug discovery and unlocking novel disease-modifying treatments for untreatable or difficult-to-treat disorders including neurodegenerative diseases, rare genetic disorders and oncology. Forward-Looking StatementsThis release contains “forward-looking statements” made pursuant to the safe harbor provisions of the Private Securities Litigation Reform Act of 1995. These statements are typically preceded by words such as “believes,” “expects,” “anticipates,” “intends,” “will,” “may,” “should,” or similar expressions. These forward-looking statements reflect management’s current knowledge, assumptions, judgment and expectations regarding future performance or events. Although management believes that the expectations reflected in such statements are reasonable, they give no assurance that such expectations will prove to be correct or that those goals will be achieved, and you should be aware that actual results could differ materially from those contained in the forward-looking statements. Forward-looking statements are subject to a number of risks and uncertainties, including, but not limited to, risks associated with market conditions and the satisfaction of customary closing conditions related to the offering and uncertainties related to the offerings and the use of proceeds from the offerings. For a further description of the risks and uncertainties that could cause actual results to differ from those expressed in these forward-looking statements, as well as risks relating to the Company’s business in general, please refer to the Company’s prospectus supplement to be filed with the SEC, and the documents incorporated by reference therein, including the Company’s Form 10-K for the year ended December 31, 2023 and Form 10-Q for the quarter ended June 30, 2024. All forward-looking statements are expressly qualified in their entirety by this cautionary notice. You are cautioned not to place undue reliance on any forward-looking statements, which speak only as of the date of this release. We have no obligation, and expressly disclaim any obligation, to update, revise or correct any of the forward-looking statements, whether as a result of new information, future events or otherwise. Investor Contacts:Apaar Jammu and Chuck Padalaajammu@gaintherapeutics.comchuck@lifesciadvisors.com Media Contacts:Russo PartnersNic Johnson and Elio Ambrosionic.johnson@russopartnersllc.comelio.ambrosio@russopartnersllc.com(760) 846-9256
Phase 1
Analysis
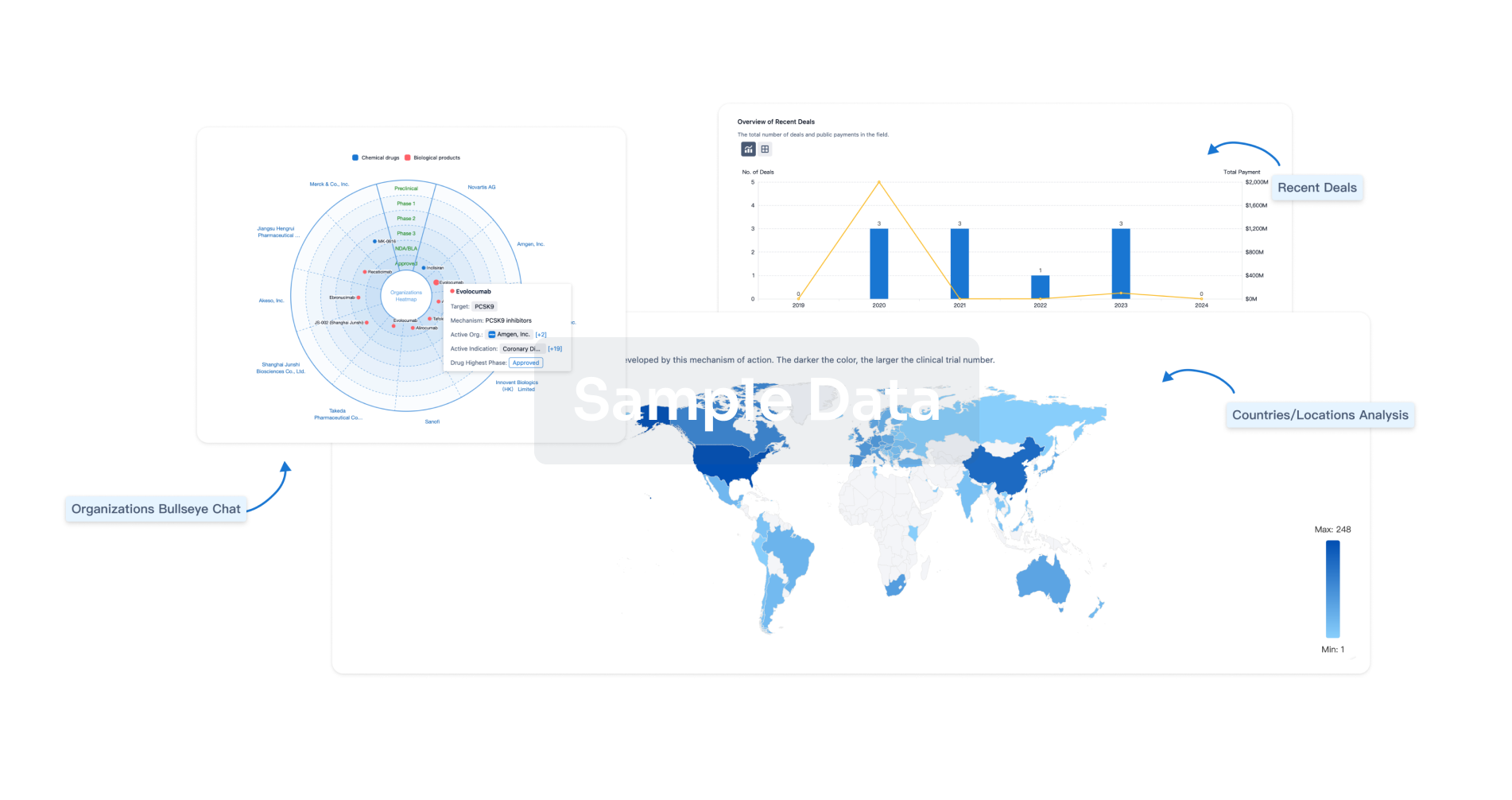
Perform a panoramic analysis of this field.
login
or
AI Agents Built for Biopharma Breakthroughs
Accelerate discovery. Empower decisions. Transform outcomes.
Get started for free today!
Accelerate Strategic R&D decision making with Synapse, PatSnap’s AI-powered Connected Innovation Intelligence Platform Built for Life Sciences Professionals.
Start your data trial now!
Synapse data is also accessible to external entities via APIs or data packages. Empower better decisions with the latest in pharmaceutical intelligence.
Bio
Bio Sequences Search & Analysis
Sign up for free
Chemical
Chemical Structures Search & Analysis
Sign up for free




