Request Demo
Last update 08 May 2025
AURK x PLK
Last update 08 May 2025
Related
1
Drugs associated with AURK x PLKTarget |
Mechanism PLK inhibitors [+1] |
Active Org.- |
Originator Org. |
Active Indication- |
Inactive Indication |
Drug Highest PhasePending |
First Approval Ctry. / Loc.- |
First Approval Date20 Jan 1800 |
100 Clinical Results associated with AURK x PLK
Login to view more data
100 Translational Medicine associated with AURK x PLK
Login to view more data
0 Patents (Medical) associated with AURK x PLK
Login to view more data
572
Literatures (Medical) associated with AURK x PLK01 May 2025·Journal of Mass Spectrometry
Multi‐Omic Evaluation of PLK1 Inhibitor—Onvansertib—In Colorectal Cancer Spheroids
Article
Author: Fries, Brian D. ; Sekera, Emily R. ; Hummon, Amanda B. ; Holbrook, Joseph H.
01 Apr 2025·Cancer Science
Article
Author: Mae, Hirokazu ; Imura, Yoshinori ; Inoue, Akitomo ; Kotani, Yuki ; Nakai, Sho ; Takami, Haruna ; Outani, Hidetatsu ; Okada, Seiji
25 Feb 2025·Proceedings of the National Academy of Sciences
A limitation lifted: A conditional knockdown system reveals essential roles for Polo-like kinase and Aurora kinase 1 in
Trypanosoma cruzi
cell division
Article
Author: Wiedeman, Justin ; Harrison, Ruby ; Etheridge, Ronald Drew
2
News (Medical) associated with AURK x PLK05 Mar 2024
SOUTH SAN FRANCISCO, Calif. and SAN DIEGO, March 05, 2024 (GLOBE NEWSWIRE) -- ORIC Pharmaceuticals, Inc. (Nasdaq: ORIC), a clinical stage oncology company focused on developing treatments that address mechanisms of therapeutic resistance, today announced that multiple abstracts have been accepted for presentation, including two oral presentations on ORIC-944, a potent and selective allosteric inhibitor of PRC2, at the 2024 American Association for Cancer Research (AACR) Annual Meeting taking place April 5-10, 2024, in San Diego, CA. Invited speaker presentation details:Title:Discovery of ORIC-944, a novel inhibitor of PRC2 with best-in-class properties for the treatment of prostate cancerSession Title:New Drugs on the Horizon: Part 1Date & Time:Sunday, April 7, 2024, 1:00 p.m. - 2:30 p.m. PTPresenter:Lori Friedman, Ph.D., Chief Scientific OfficerAbstract:Embargoed until April 7, 2024 Oral presentation details:Title:ORIC-944, a potent and selective allosteric PRC2 inhibitor with best-in class properties, demonstrates combination synergy with AR pathway inhibitors in prostate cancer modelsAbstract Number:6856Date & Time:Tuesday, April 9, 2024, 2:30 p.m. - 4:30 p.m. PTSession Category:Experimental and Molecular TherapeuticsSession Title:Novel Antitumor Agents 5Presenter:Anneleen Daemen, Ph.D., Executive Director of Translational Medicine Abstract Highlights ORIC-944, a potent, highly selective, orally bioavailable inhibitor of PRC2 demonstrated single agent tumor growth inhibition in a spectrum of AR-positive in vivo prostate cancer models, including those expressing AR mutants or ARv7. Combining ORIC-944 with an AR inhibitor was synergistic in multiple prostate cancer cell line models, and combination efficacy for ORIC-944 with AR inhibition was confirmed in vivo. RNA-seq analysis of transcriptional changes induced by ORIC-944 provided mechanistic insight into the role of PRC2 in prostate cancer lineage plasticity and combination response. These results position ORIC-944 as a potential best-in-class PRC2 inhibitor for combination with AR inhibitors in patients with prostate cancer. Poster presentation details:Title:ORIC-613, a potential first- and best-in-class, orally bioavailable, potent and selective PLK4 inhibitor with synthetic lethality in TRIM37 high cancer modelsAbstract Number:594Date & Time:Sunday April 7, 2024, 1:30 p.m. - 5:00 p.m. PTSession Category:Experimental and Molecular TherapeuticsSession Title:Kinase and Phosphatase Inhibitors 1Location:Poster Section 25 Abstract Highlights ORIC-613 is an orally bioavailable, potent and exquisitely selective small molecule inhibitor of PLK4, which is synthetic lethal in tumor cells with high levels of TRIM37. ORIC-613 is highly selective against the kinome, including against the closely related aurora kinases and other PLK family members. Preclinical assessment in cancer cell lines revealed the synthetic lethality, with ORIC-613 having stronger potency in TRIM37-high cells as evidenced by inducing tumor cell death specifically in TRIM37-high versus TRIM37-wildtype cells. Analysis of genomic data from adult tumors indicates that increased TRIM37 copy number is found across a breadth of cancers, with notable prevalence in breast cancer. Oral dosing of ORIC-613 resulted in tumor growth inhibition and regressions in TRIM37-high xenograft breast tumors. These results position ORIC-613 as a potential first- and best-in-class development candidate, which demonstrates synthetic lethality in TRIM37-high tumors and has the potential to benefit these patients. All regular abstracts are available for viewing via AACR’s online itinerary planner located, here. Invited speaker abstracts will be available for viewing the morning of their associated session. About ORIC Pharmaceuticals, Inc. ORIC Pharmaceuticals is a clinical stage biopharmaceutical company dedicated to improving patients’ lives by Overcoming Resistance In Cancer. ORIC’s clinical stage product candidates include (1) ORIC-114, a brain penetrant inhibitor designed to selectively target EGFR and HER2 with high potency against exon 20 insertion mutations, being developed across multiple genetically defined cancers, (2) ORIC-944, an allosteric inhibitor of the polycomb repressive complex 2 (PRC2) via the EED subunit, being developed for prostate cancer, and (3) ORIC-533, an orally bioavailable small molecule inhibitor of CD73, a key node in the adenosine pathway believed to play a central role in resistance to chemotherapy- and immunotherapy-based treatment regimens, being developed for multiple myeloma. Beyond these three product candidates, ORIC is also developing multiple precision medicines targeting other hallmark cancer resistance mechanisms. ORIC has offices in South San Francisco and San Diego, California. For more information, please go to www.oricpharma.com, and follow us on X or LinkedIn. Cautionary Note Regarding Forward-Looking StatementsThis press release contains forward-looking statements as that term is defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934. Statements in this press release that are not purely historical are forward-looking statements. Such forward-looking statements include, among other things, statements regarding the potential best-in-class properties of ORIC-944; ORIC-613 as a potential first- and best-in-class development candidate; the development plans for ORIC-944 and ORIC’s other product candidates; the potential advantages of ORIC-944, ORIC-613 and ORIC’s other product candidates and programs; and plans underlying ORIC’s clinical trials and development. Words such as “believes,” “anticipates,” “plans,” “expects,” “intends,” “will,” “goal,” “potential” and similar expressions are intended to identify forward-looking statements. The forward-looking statements contained herein are based upon ORIC’s current expectations and involve assumptions that may never materialize or may prove to be incorrect. Actual results could differ materially from those projected in any forward-looking statements due to numerous risks and uncertainties, including but not limited to: risks associated with the process of discovering, developing and commercializing drugs that are safe and effective for use as human therapeutics and operating as an early clinical stage company; ORIC’s ability to develop, initiate or complete preclinical studies and clinical trials for, obtain approvals for and commercialize any of its product candidates; changes in ORIC’s plans to develop and commercialize its product candidates; the potential for clinical trials of ORIC’s product candidates to differ from preclinical, initial, interim, preliminary or expected results; negative impacts of health emergencies, economic instability or international conflicts on ORIC’s operations, including clinical trials; the risk of the occurrence of any event, change or other circumstance that could give rise to the termination of ORIC’s license and collaboration agreements; the potential market for ORIC’s product candidates, and the progress and success of competing therapeutics currently available or in development; ORIC’s ability to raise any additional funding it will need to continue to pursue its business and product development plans; regulatory developments in the United States and foreign countries; ORIC’s reliance on third parties, including contract manufacturers and contract research organizations; ORIC’s ability to obtain and maintain intellectual property protection for its product candidates; the loss of key scientific or management personnel; competition in the industry in which ORIC operates; general economic and market conditions; and other risks. Information regarding the foregoing and additional risks may be found in the section entitled “Risk Factors” in ORIC’s Quarterly Report on Form 10-Q filed with the Securities and Exchange Commission (the “SEC”) on November 6, 2023, and ORIC’s future reports to be filed with the SEC. These forward-looking statements are made as of the date of this press release, and ORIC assumes no obligation to update the forward-looking statements, or to update the reasons why actual results could differ from those projected in the forward-looking statements, except as required by law. Contact:Dominic Piscitelli, Chief Financial Officerdominic.piscitelli@oricpharma.cominfo@oricpharma.com
AACR
19 Apr 2023
SOUTH SAN FRANCISCO, Calif. and SAN DIEGO, April 18, 2023 (GLOBE NEWSWIRE) -- ORIC Pharmaceuticals, Inc. (Nasdaq: ORIC), a clinical stage oncology company focused on developing treatments that address mechanisms of therapeutic resistance, presented two preclinical poster presentations at the 2023 American Association for Cancer Research (AACR) Annual Meeting. “Our presentations at AACR reflect the excellent preclinical scientific support of our development pipeline,” said Lori Friedman, PhD, chief scientific officer. “Preclinical data provided insights into the comprehensive biomarker strategy for ORIC-944, our PRC2 inhibitor in Phase 1 as a monotherapy for metastatic prostate cancer. We are also pleased with the progress of our PLK4 program, with data showing that the exquisite kinase selectivity is key to synthetic lethality in tumor models with high TRIM37.” Presentation details: ORIC-944: allosteric inhibitor of PRC2 ORIC-944 is a potent, orally bioavailable, highly selective allosteric small molecule inhibitor of PRC2, the complex which tri-methylates histone H3 lysine 27 (H3K27me3) via targeting the embryonic ectoderm development (EED) subunit, and is in early clinical development as a monotherapy for patients with metastatic prostate cancer. Poster Presentation: Biomarker strategy for a phase 1 study of ORIC-944, a potent and selective allosteric PRC2 inhibitor, in patients with metastatic prostate cancer
Key findings of the presentation: Preclinical pharmacology studies in mice showed that ORIC-944 induces dose- and time-dependent H3K27me3 reduction in the skin epidermis, peripheral blood monocytes, and cell free nucleosomes in plasma, with the latter PD modulation being tumor-specific.Putative PRC2 target genes were identified via time-dependent transcriptional and epigenetic profiling of xenograft tumor models orally dosed with ORIC-944.These studies and corresponding assay development support the comprehensive biomarker strategy of target engagement and pharmacodynamic biomarkers implemented in the ongoing Phase 1 trial of ORIC-944 in patients with metastatic prostate cancer. PLK4 Inhibitor ProgramThe PLK4 inhibitor program is a small molecule therapeutic program intended to address a mechanism of innate resistance found in a subset of breast cancers, specifically a synthetic lethal interaction of polo-like kinase 4 (PLK4) inhibition in tumors bearing a TRIM37 DNA amplification/elevation. A novel, potent, highly selective, orally bioavailable PLK4 inhibitor was selected as a development candidate in the fourth quarter of 2022. Poster Presentation: Selective PLK4 inhibition demonstrates synthetic lethality in TRIM37 amplified neuroblastoma and breast cancer models while less selective inhibitors do not
Key findings of the presentation: ORIC discovered novel, potent, orally bioavailable small molecule inhibitors of PLK4 that are highly selective, including against the closely related aurora kinases and PLK1-3.ORIC PLK4 selective inhibitors show greater potency in TRIM37 high cancer cell lines relative to low TRIM37 low cell lines, whereas no differential was observed for non-selective inhibitors.Apoptotic cell death was observed solely in TRIM37 high cancer cells for ORIC PLK4 selective inhibitors, in contrast to non-selective inhibitors which did not show this synthetic lethality.Using a genetically engineered PLK4 cell line variant (PLK4 G95L) that prevents compound-mediated inhibition of PLK4, selective PLK4 inhibitors lose activity in TRIM37 high G95L cells, confirming on-target cell activity, compared with non-selective inhibitors whose cell potency is not dependent on PLK4 inhibition.PLK4 inhibition blocks trans-autophosphorylation and PLK4 protein degradation and correlates with cell viability for selective compounds, and not for non-selective inhibitors.Together these mechanistic data confirm the potential of highly selective PLK4 inhibition as a synthetic lethal therapy for TRIM37 amplified/elevated cancers. About ORIC Pharmaceuticals, Inc. ORIC Pharmaceuticals is a clinical stage biopharmaceutical company dedicated to improving patients’ lives by Overcoming Resistance In Cancer. ORIC’s clinical stage product candidates include (1) ORIC-533, an orally bioavailable small molecule inhibitor of CD73, a key node in the adenosine pathway believed to play a central role in resistance to chemotherapy- and immunotherapy-based treatment regimens, being developed for multiple myeloma, (2) ORIC-114, a brain penetrant inhibitor designed to selectively target EGFR and HER2 with high potency against exon 20 insertion mutations, being developed across multiple genetically defined cancers, and (3) ORIC-944, an allosteric inhibitor of the polycomb repressive complex 2 (PRC2) via the EED subunit, being developed for prostate cancer. Beyond these three product candidates, ORIC is also developing multiple precision medicines targeting other hallmark cancer resistance mechanisms. ORIC has offices in South San Francisco and San Diego, California. For more information, please go to www.oricpharma.com, and follow us on Twitter or LinkedIn. Cautionary Note Regarding Forward-Looking StatementsThis press release contains forward-looking statements as that term is defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934. Statements in this press release that are not purely historical are forward-looking statements. Such forward-looking statements include, among other things, statements regarding the advantages of ORIC’s biomarker strategy for ORIC-944; plans underlying ORIC’s preclinical PLK4 inhibitor program; the potential advantages of ORIC’s product candidates and programs; plans underlying the clinical or preclinical development of any of ORIC’s other programs; and statements by the company’s chief scientific officer. Words such as “believes,” “anticipates,” “plans,” “expects,” “intends,” “will,” “goal,” “potential” and similar expressions are intended to identify forward-looking statements. The forward-looking statements contained herein are based upon ORIC’s current expectations and involve assumptions that may never materialize or may prove to be incorrect. Actual results could differ materially from those projected in any forward-looking statements due to numerous risks and uncertainties, including but not limited to: risks associated with the process of discovering, developing and commercializing drugs that are safe and effective for use as human therapeutics and operating as an early clinical stage company; ORIC’s ability to develop, initiate or complete preclinical studies and clinical trials for, obtain approvals for and commercialize any of its product candidates; changes in ORIC’s plans to develop and commercialize its product candidates; the potential for clinical trials of ORIC-533, ORIC-114, ORIC-944 or any other product candidates to differ from preclinical, initial, interim, preliminary or expected results; negative impacts of the COVID-19 pandemic on ORIC’s operations, including clinical trials; the risk of the occurrence of any event, change or other circumstance that could give rise to the termination of ORIC’s license and collaboration agreements; ORIC’s ability to raise any additional funding it will need to continue to pursue its business and product development plans; regulatory developments in the United States and foreign countries; ORIC’s reliance on third parties, including contract manufacturers and contract research organizations; ORIC’s ability to obtain and maintain intellectual property protection for its product candidates; the loss of key scientific or management personnel; competition in the industry in which ORIC operates; general economic and market conditions; and other risks. Information regarding the foregoing and additional risks may be found in the section entitled “Risk Factors” in ORIC’s Annual Report on Form 10-K filed with the Securities and Exchange Commission (the “SEC”) on March 16, 2023, and ORIC’s future reports to be filed with the SEC. These forward-looking statements are made as of the date of this press release, and ORIC assumes no obligation to update the forward-looking statements, or to update the reasons why actual results could differ from those projected in the forward-looking statements, except as required by law. Contact:Dominic Piscitelli, Chief Financial Officerdominic.piscitelli@oricpharma.com info@oricpharma.com
Phase 1AACRImmunotherapy
Analysis
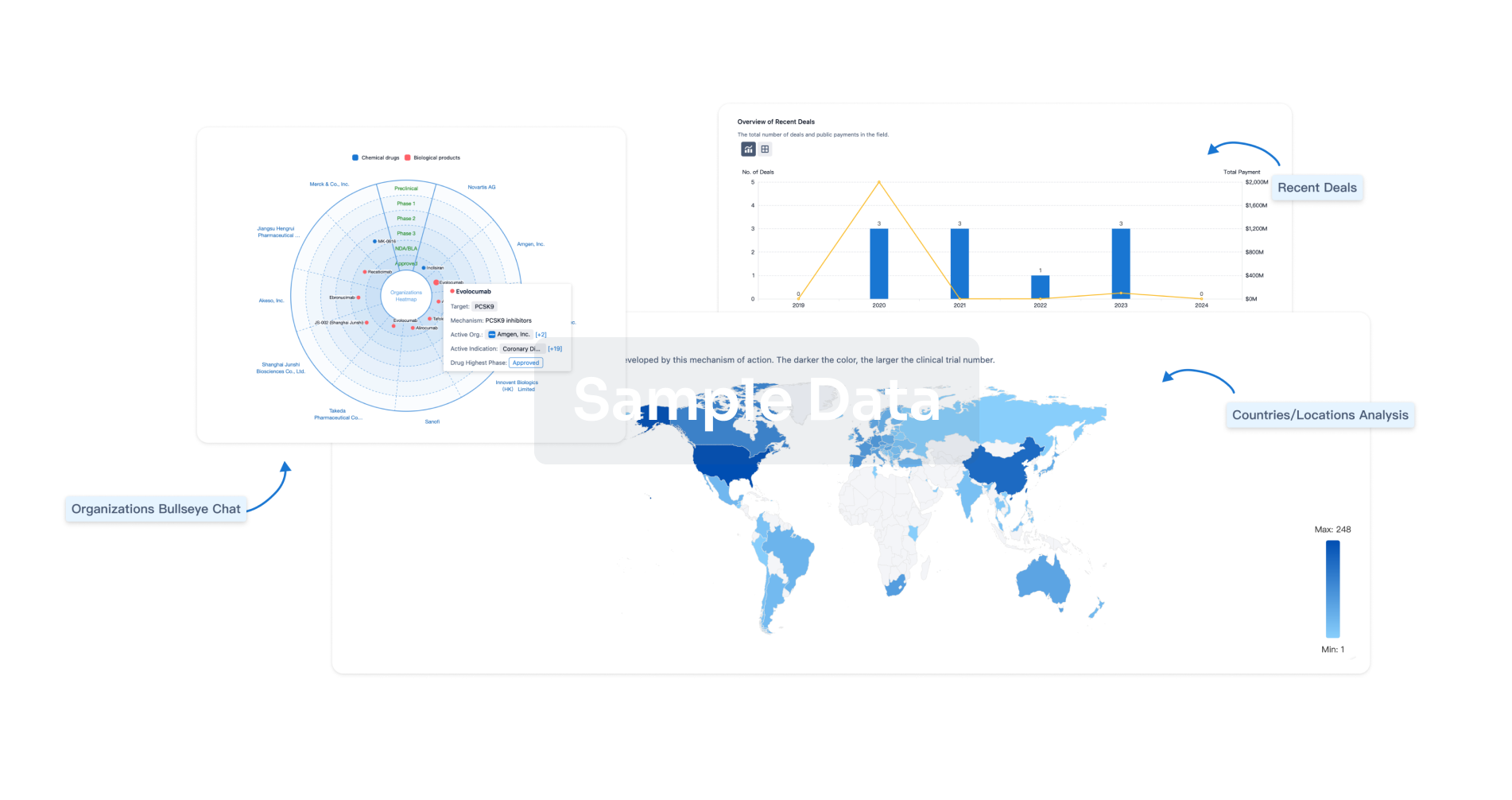
Perform a panoramic analysis of this field.
login
or
AI Agents Built for Biopharma Breakthroughs
Accelerate discovery. Empower decisions. Transform outcomes.
Get started for free today!
Accelerate Strategic R&D decision making with Synapse, PatSnap’s AI-powered Connected Innovation Intelligence Platform Built for Life Sciences Professionals.
Start your data trial now!
Synapse data is also accessible to external entities via APIs or data packages. Empower better decisions with the latest in pharmaceutical intelligence.
Bio
Bio Sequences Search & Analysis
Sign up for free
Chemical
Chemical Structures Search & Analysis
Sign up for free
