Request Demo
Last update 08 May 2025
TCF7L2
Last update 08 May 2025
Basic Info
Synonyms HMG box transcription factor 4, hTCF-4, T-cell factor 4 + [6] |
Introduction Participates in the Wnt signaling pathway and modulates MYC expression by binding to its promoter in a sequence-specific manner. Acts as a repressor in the absence of CTNNB1, and as activator in its presence. Activates transcription from promoters with several copies of the Tcf motif 5'-CCTTTGATC-3' in the presence of CTNNB1. TLE1, TLE2, TLE3 and TLE4 repress transactivation mediated by TCF7L2/TCF4 and CTNNB1. Expression of dominant-negative mutants results in cell-cycle arrest in G1. Necessary for the maintenance of the epithelial stem-cell compartment of the small intestine. |
Related
1
Drugs associated with TCF7L2Target |
Mechanism TCF7L2 modulators |
Active Org. |
Originator Org. |
Active Indication |
Inactive Indication- |
Drug Highest PhaseDiscovery |
First Approval Ctry. / Loc.- |
First Approval Date20 Jan 1800 |
100 Clinical Results associated with TCF7L2
Login to view more data
100 Translational Medicine associated with TCF7L2
Login to view more data
0 Patents (Medical) associated with TCF7L2
Login to view more data
1,727
Literatures (Medical) associated with TCF7L201 Dec 2025·Genes & Nutrition
Changes in triglyceride-rich lipoprotein particle profiles in response to one-week on a low fat or Mediterranean diet by TCF7L2 rs7903146 genotype: a randomized crossover dietary intervention trial
Article
Author: Lai, Chao-Qiang ; Gervis, Julie E ; Parnell, Laurence D ; Ordovas, Jose M ; Lichtenstein, Alice H
01 Jun 2025·Food and Chemical Toxicology
Elaidic acid induces testicular oxidative stress, inflammation, Wnt/β-catenin disruption and abnormalities in steroidogenesis, spermatogenesis and histo-architecture in Sprague Dawley rats
Article
Author: Al-Emam, Ahmed ; Alzahrani, Khalid J ; Alsuwat, Meshari A ; Akbar, Ali ; Zahara, Syeda Sania ; Alzahrani, Fuad M ; Hayat, Muhammad Faisal
01 Jun 2025·Contemporary Clinical Trials Communications
An exploratory, open-label, parallel group, randomised controlled trial evaluating the effect of gene based nutritional intervention in type 2 diabetes: The protocol for NUDGE clinical trial
Article
Author: Kushwaha, Savitesh ; Gupta, Pramod Kumar ; Sagar, Vivek ; Khanna, Poonam ; Thakur, Jarnail Singh ; Gupta, Madhu ; Srivastava, Rachana ; Bhadada, Sanjay Kumar
2
News (Medical) associated with TCF7L205 Sep 2024
THURSDAY, Sept. 5, 2024 -- World Trade Center (WTC) exposure is associated with increased DNA methylation, which may contribute to
breast cancer
, according to a study published in the June issue of
Environmental Epidemiology
.
Stephanie Tuminello, Ph.D., M.P.H., from the NYU Grossman School of Medicine in New York City, and colleagues examined the DNA methylation profiles of WTC-exposed community members who remained
cancer
-free versus those who developed breast cancer. The study included 64 WTC-exposed women (32 cancer-free, 32 with breast cancer) and 32 WTC-unexposed women from the NYU Women's Health Study (16 cancer-free and 16 with prediagnostic breast cancer).
The researchers found that compared with unexposed participants, WTC-exposed women more often had hypermethylated cytosine-phosphate-guanine probe sites (14.3 versus 4.5 percent). In WTC-exposed groups (breast cancer patients and cancer-free individuals), cancer-related pathways were overrepresented. Forty-seven epigenetically dysregulated genes were identified among WTC-exposed breast cancers compared with the unexposed breast cancer patients. A network was formed from these genes, including Wnt/β-catenin signaling genes
WNT4
and
TCF7L2
; dysregulation of these genes contributed to cancer immune evasion.
"WTC exposure is associated with global and site-specific DNA methylation changes. This was observed among cancer-free WTC-exposed survivors as well as those with breast cancer," the authors write. "Several cancer-related genes and pathways appear to be impacted, and, specifically, WTC exposure may compromise the ability of the immune system to identify and eliminate cancer cells contributing to cancer immune evasion."
Abstract/Full Text
Whatever your topic of interest,
subscribe to our newsletters
to get the best of Drugs.com in your inbox.
Clinical ResultClinical Study
30 Aug 2022
A growing body of research suggests that the disease may also flow from defects in glia, important support cells found in the brain. The new study expands our understanding of the underlying mechanisms of the disease, and reinforces the potential of therapies that target glia cells.
Huntington's disease -- a hereditary and fatal genetic disorder -- has long been considered a neuronal disease due to the permanent loss of medium spiny motor neurons, the death of which over time is responsible for the clinical hallmarks of the disease: involuntary movements, problems with coordination, cognitive decline, depression, and psychosis.
However, a growing body of research, including a new study appearing in the journal Cell Reports, suggests that the disease may also flow from defects in glia, important support cells found in the brain. The new study expands our understanding of the underlying mechanisms of the disease, and reinforces the potential of therapies that target glia cells.
Years of research in the lab of University of Rochester Medical Center (URMC) neurologist Steve Goldman, M.D., Ph.D., have shown that the two populations of glia found in the brain -- astrocytes and oligodendrocytes -- are dysfunctional in Huntington's disease, and may trigger much of the neuronal pathology seen in the disease. Goldman is co-director of the URMC Center for Translational Neuromedicine and senior author of the new study. Glia cells play a critical role in maintaining the health of neurons and facilitating the chemical signaling between nerve cells. In Huntington's, glia are unable to perform these functions, leading to a breakdown in communication between neurons and, over time, cell death.
"Huntington's is a complex disease that impacts both neurons and support cells. To use an analogy, not only is the patient sick, but so are the doctor and the nurse," said Abdellatif Benraiss, Ph.D., a research associate professor in the URCM Department of Neurology and first author of the study. "While the loss of neurons gives rise to the symptoms and the ultimate fatal nature of the disease, reversing glial dysfunction may give us an opportunity to intervene early in the course of the disease, keeping neurons healthy for longer and slowing disease progression."
The new study focuses on oligodendrocytes and identifies how the suppression of a specific transcription gene called Tcf7l2 triggers a series of changes that impair the function of oligodendrocyte progenitor cells (OPCs). These cells constantly resupply the brain with oligodendrocytes, which, in turn, refresh the myelin insulation that helps signals travel in the brain more crisply. In Huntington's, OPCs are not able to meet demand, leading to deficient myelination in the brain, which can be observed in Huntington's patients in the form of white matter atrophy. When the researchers overexpressed Tcf7l2 in mice with the Huntington's disease mutation, their OPCs recovered and restored the myelin that had been lost to the disease.
A sister paper from the Goldman lab, which appeared in Cell Reports last year, examined how the genetic defect that lies at the heart of the disease impacts the development and function of astrocytes, which support neurons and their synaptic connections. That paper highlighted genetic pathways aligned with Tcf7l2, found in both mouse and human Huntington's astrocytes, that is a major contributor to synaptic dysfunction in Huntington's, which in turn leads to the behavioral and psychiatric symptoms of the disease. Taken together, these papers provide a clearer picture of the genetic mechanisms by which Huntington's disease impairs glial cell function and ultimately leads to neurological disability, while providing new cellular and molecular targets for potential treatment.
The researchers believe that these findings put new therapies within reach. Replacing or "fixing" defective glia cells may prove a far easier proposition than replenishing neurons lost in the disease. A study from Goldman's lab in 2018 showed the complexity of the genetic defects in Huntington's glia, and pointed to the utility of swapping out sick cells with healthy ones, an approach that the lab had shown effective in mouse models of the disease in an earlier study in 2016. Taken together, this series of studies has laid the foundation for targeting glial cells for treatment, and potentially outright replacement, in Huntington disease.
Additional authors of the study include John Mariani, Ashley Tate, Pernille Madsen, Kathleen Clark, Kevin Welle, Renee Solly, Laetitian Capellano, Karen Bentley, and Devin Chandler-Militello with URMC. The research was funded with support from National Institute of Neurological Disorders and Stroke, the Hereditary Disease Foundation, CHDI, and Sana Biotechnology.
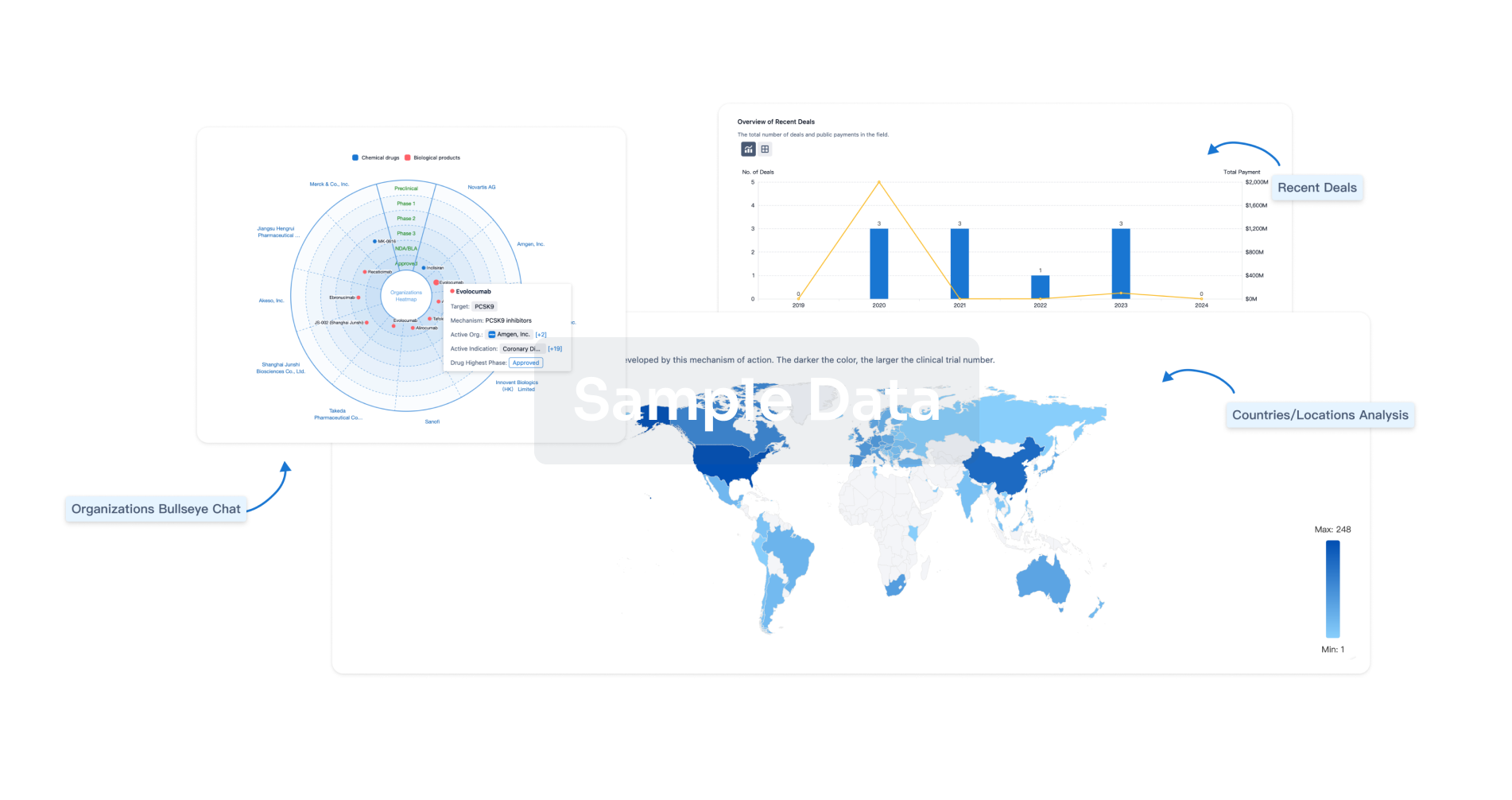
Analysis
Perform a panoramic analysis of this field.
login
or
AI Agents Built for Biopharma Breakthroughs
Accelerate discovery. Empower decisions. Transform outcomes.
Get started for free today!
Accelerate Strategic R&D decision making with Synapse, PatSnap’s AI-powered Connected Innovation Intelligence Platform Built for Life Sciences Professionals.
Start your data trial now!
Synapse data is also accessible to external entities via APIs or data packages. Empower better decisions with the latest in pharmaceutical intelligence.
Bio
Bio Sequences Search & Analysis
Sign up for free
Chemical
Chemical Structures Search & Analysis
Sign up for free
