Request Demo
Last update 08 May 2025
GRK2 x ROCK1
Last update 08 May 2025
Related
1
Drugs associated with GRK2 x ROCK1Target |
Mechanism GRK2 inhibitors [+1] |
Active Org.- |
Originator Org. |
Active Indication- |
Inactive Indication |
Drug Highest PhasePending |
First Approval Ctry. / Loc.- |
First Approval Date20 Jan 1800 |
100 Clinical Results associated with GRK2 x ROCK1
Login to view more data
100 Translational Medicine associated with GRK2 x ROCK1
Login to view more data
0 Patents (Medical) associated with GRK2 x ROCK1
Login to view more data
5
Literatures (Medical) associated with GRK2 x ROCK101 Dec 2017·Molecular PharmacologyQ3 · MEDICINE
Structural Determinants Influencing the Potency and Selectivity of Indazole-Paroxetine Hybrid G Protein–Coupled Receptor Kinase 2 Inhibitors
Q3 · MEDICINE
Article
Author: Koch, Walter J ; Waldschmidt, Helen V ; Cheung, Joseph Y ; Tesmer, John J G ; Cannavo, Alessandro ; Bouley, Renee ; Cato, M Claire ; Larsen, Scott D ; Song, Jianliang ; Yao, Xin-Qiu
28 Apr 2016·Journal of Medicinal ChemistryQ1 · MEDICINE
Structure-Based Design, Synthesis, and Biological Evaluation of Highly Selective and Potent G Protein-Coupled Receptor Kinase 2 Inhibitors
Q1 · MEDICINE
Article
Author: Joseph Y. Cheung ; Kelly M. Larimore ; Osvaldo Cruz-Rodríguez ; Helen V. Waldschmidt ; John J. G. Tesmer ; Walter J. Koch ; Jianliang Song ; Paul D. Kirchhoff ; Marilyn C. Cato ; Jessica Waninger-Saroni ; Alessandro Cannavo ; Scott D. Larsen ; Kristoff T. Homan
01 Jun 2013·Hepatobiliary & Pancreatic Diseases InternationalQ3 · MEDICINE
Desensitization of G-protein-coupled receptors induces vascular hypocontractility in response to norepinephrine in the mesenteric arteries of cirrhotic patients and rats
Q3 · MEDICINE
Article
Author: Jun Qin ; De-Jun Liu ; Jia Xu ; Wei Chen ; Jiang-Yong Sang ; Yan-Miao Huo ; Zhi-Yong Wu
Analysis
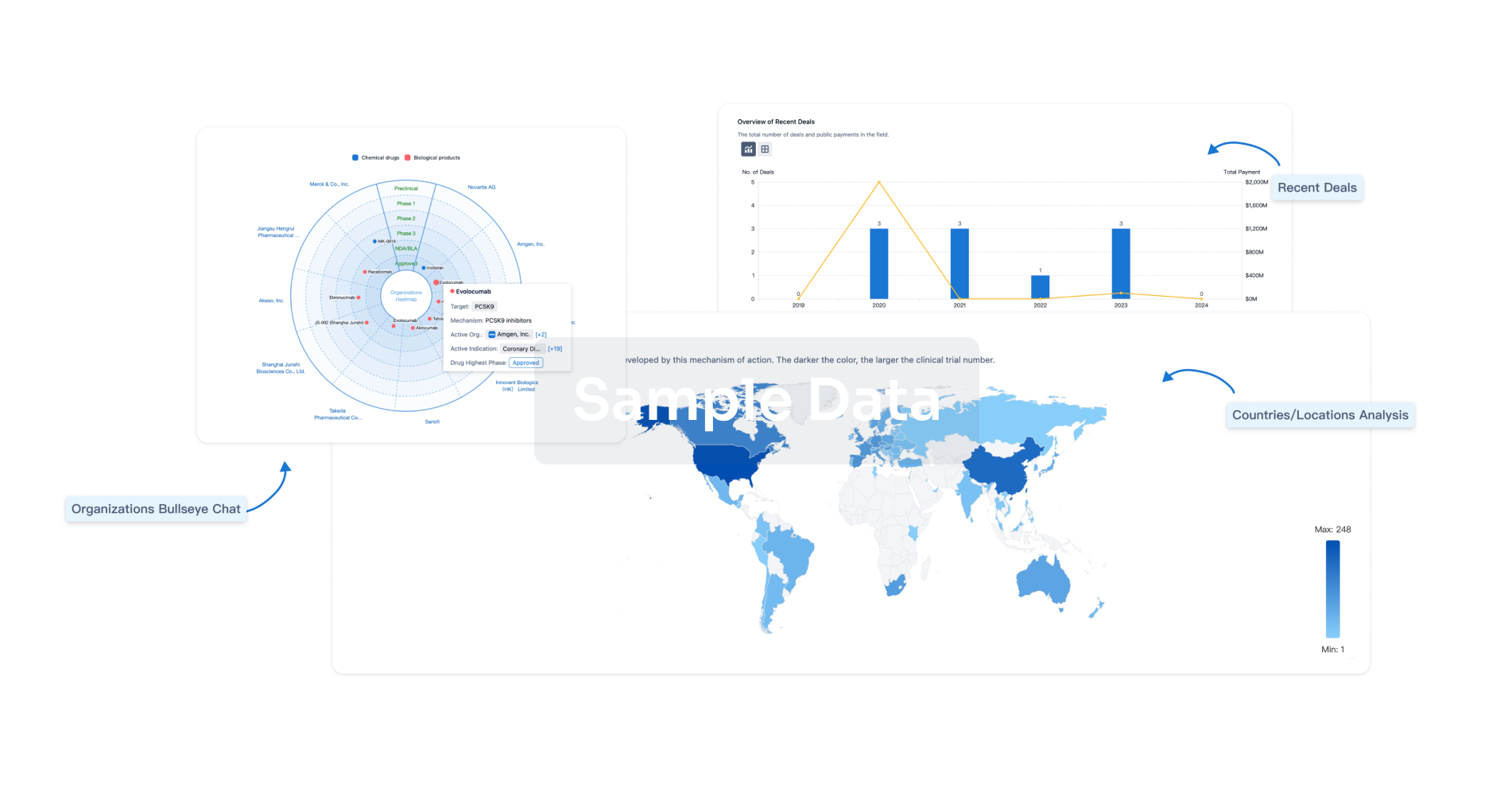
Perform a panoramic analysis of this field.
login
or
AI Agents Built for Biopharma Breakthroughs
Accelerate discovery. Empower decisions. Transform outcomes.
Get started for free today!
Accelerate Strategic R&D decision making with Synapse, PatSnap’s AI-powered Connected Innovation Intelligence Platform Built for Life Sciences Professionals.
Start your data trial now!
Synapse data is also accessible to external entities via APIs or data packages. Empower better decisions with the latest in pharmaceutical intelligence.
Bio
Bio Sequences Search & Analysis
Sign up for free
Chemical
Chemical Structures Search & Analysis
Sign up for free