Enhancing Oral Azacitidine Efficacy with Cedazuridine in a Mouse Model: Bridging the Gap to Intravenous Therapy
A recent development combined DAC with cedazuridine (CDZ), an oral CDA inhibitor, in a fixed-dose combination tablet (ASTX727) to mimic the pharmacokinetics of IV DAC, as reported in Lancet Haematology 2019. To explore a similar approach with AZA, researchers investigated the possibility of enhancing the bioavailability of oral AZA with CDZ in a mouse tumor model.
The study involved measuring GI50 in AML cell lines treated with AZA, CDZ, or their combination. Despite cancer cell lines producing CDA, the levels are minimal compared to those in the gut and liver. Therefore, a systemic model of AML in immunodeficient mice was used, where mice were irradiated and injected with MOLM-13 AML cells. The mice were then randomly assigned to receive AZA via intraperitoneal (i.p.) injection, oral AZA, CDZ plus oral AZA, or CDZ alone. The treatments were administered daily for seven days.
The study found no significant difference in cell viability between AZA and the AZA/CDZ combination in vitro. However, in the xenograft model, i.p. AZA significantly reduced leukemic expansion in the bone marrow and spleen, while CDZ alone had no effect. Notably, oral AZA alone did not decrease AML expansion, but when combined with CDZ, it significantly reduced AML in both bone marrow and spleen. Importantly, the combination of CDZ and oral AZA was as effective as traditional i.p. AZA dosing in bone marrow and splenic tissue.
Survival analysis showed that mice treated with CDZ alone died within 21 days post-transplant, whereas those treated with i.p. AZA or oral AZA plus CDZ had a 50% extended survival, with no significant difference between the two treatment groups. H&E staining indicated no significant bone marrow toxicity, suggesting AZA's selective effect on transplanted MOLM-13 AML cells.
These findings support the potential of oral AZA combined with CDA-inhibitor CDZ as a viable alternative to traditional AZA treatment, providing a basis for the development of a fixed-dose combination therapy (ASTX030) for myeloid diseases. Clinical trials for ASTX030 are in the planning stages.
How to Use Synapse Database to Search and Analyze Translational Medicine Data?
The transational medicine section of the Synapse database supports searches based on fields such as drug, target, and indication, covering the T0-T3 stages of translation. Additionally, it offers a historical conference search function as well as filtering options, view modes, translation services, and highlights summaries, providing you with a unique search experience.
Taking obesity as an example, select "obesity" under the indication category and click search to enter the Translational Medicine results list page. By clicking on the title, you can directly navigate to the original page.
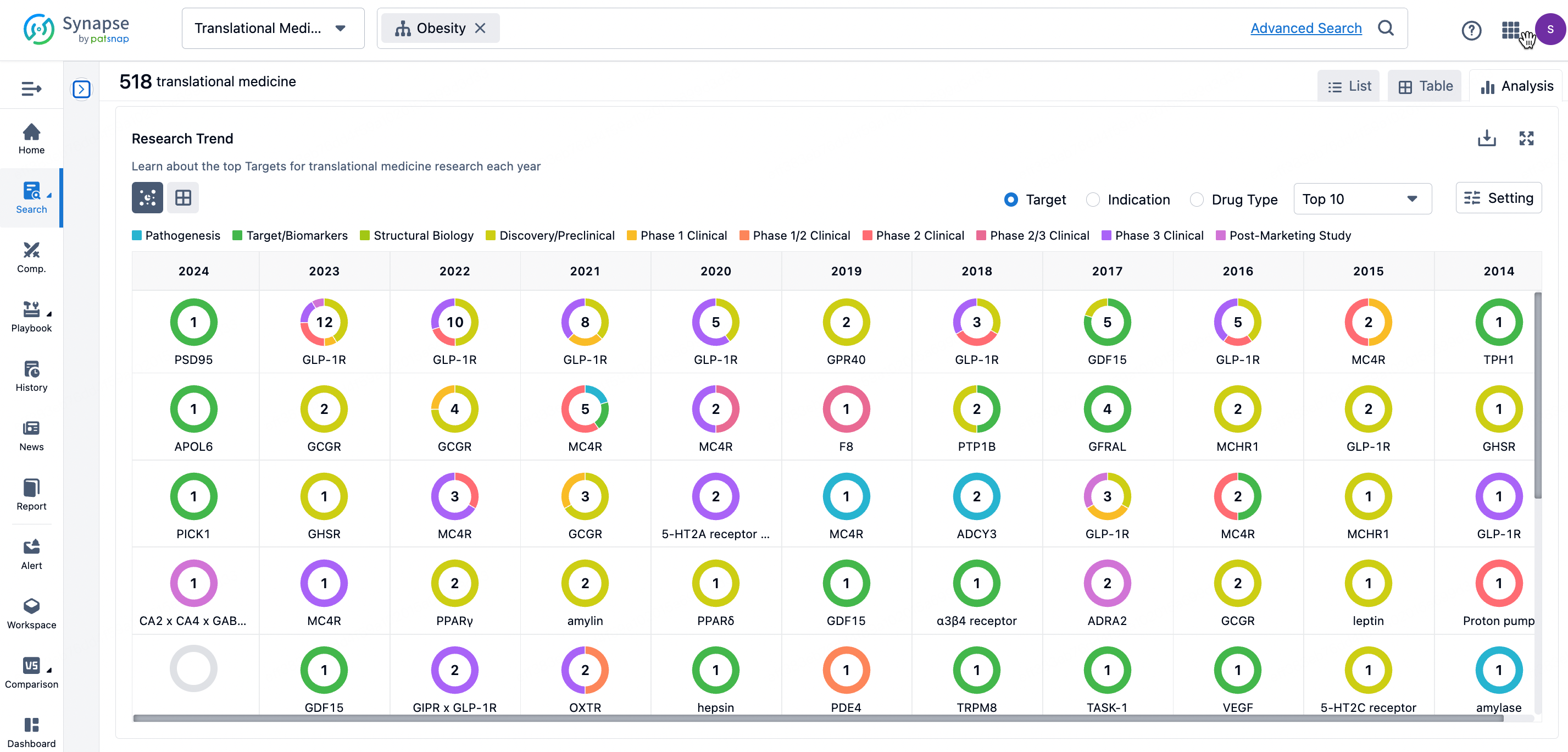
By clicking the analysis button, you can observe that GLP-1R treatment for obesity has gained significant attention over the past three years, with preclinical research still ongoing in 2023. Additionally, there are emerging potential targets, such as GDF15, among others.
Click on the image below to go directly to the Translational Medicine search interface.