Exploring the Enhanced Anticonvulsant Activity of Cyclic Sulfate Derivatives in Sugar Sulfamates: Insights from Topiramate Analogues
A key finding was the establishment of the C1 configuration as R for a primary alcohol diastereomer through single-crystal X-ray analysis, which was achieved via the selective addition of methylmagnesium bromide to an aldehyde. The study also detailed the stereoselective syntheses of hydrindane carbocyclic analogues and the synthesis of rare cyclic imidosulfites and imidosulfate, utilizing a novel sulfur dichloride reagent.
The SAR investigation led to the discovery of a potent 4,5-cyclic sulfate analogue, RWJ-37947, which demonstrated significant anticonvulsant activity in maximal electroshock seizure tests in both mice and rats, with a long duration of action exceeding 24 hours and minimal neurotoxicity. Unlike topiramate and phenytoin, this analogue did not interfere with seizures induced by certain compounds, and it was not active in various in vitro receptor binding and uptake assays. However, it was found to be a potent inhibitor of carbonic anhydrase across different rat tissue sources.
Further analysis of several analogues indicated that potent anticonvulsant activity is associated with small alkyl substituents on nitrogen and limited modifications to the cyclic sulfate group. Notably, compounds with reduced carbonic anhydrase inhibitory activity still maintained potent anticonvulsant effects, suggesting that enzyme inhibition may not be a crucial factor in the anticonvulsant activity.
Additionally, the study found that certain alpha-L-sorbopyranoses were nearly twice as potent as topiramate, and the L-fructose enantiomers of the drug showed moderate anticonvulsant activity. The log P values for topiramate and its cyclic sulfate analogue were determined to be less than optimal for CNS-active agents, yet analogues with more favorable calculated log P values did not exhibit increased potency, indicating a complex relationship between lipophilicity and anticonvulsant effectiveness.
In summary, the study provides valuable insights into the molecular features that contribute to the anticonvulsant properties of topiramate and its analogues, paving the way for the development of potentially more effective and safer antiepileptic drugs.
How to Use Synapse Database to Search and Analyze Translational Medicine Data?
The transational medicine section of the Synapse database supports searches based on fields such as drug, target, and indication, covering the T0-T3 stages of translation. Additionally, it offers a historical conference search function as well as filtering options, view modes, translation services, and highlights summaries, providing you with a unique search experience.
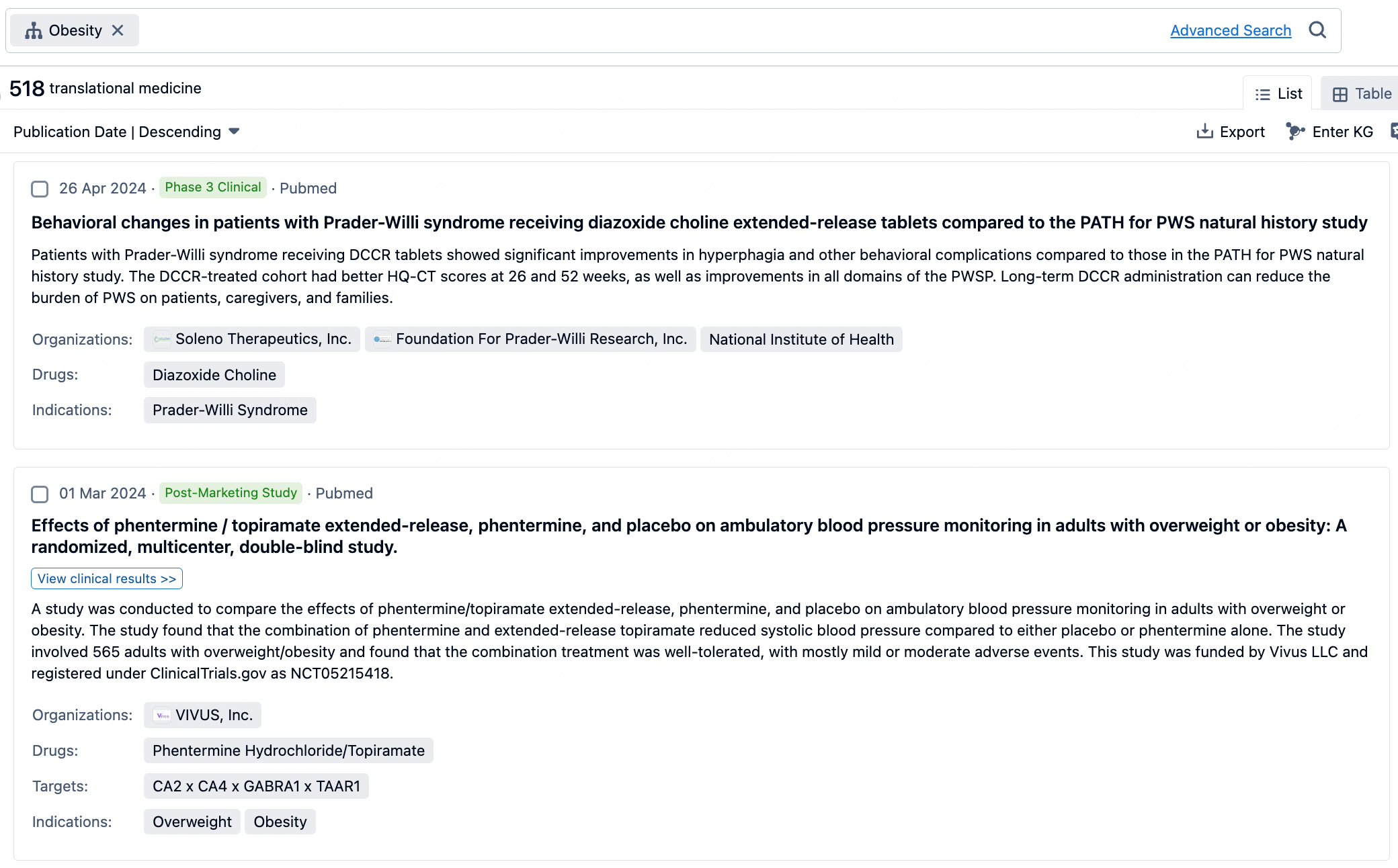
Taking obesity as an example, select "obesity" under the indication category and click search to enter the Translational Medicine results list page. By clicking on the title, you can directly navigate to the original page.
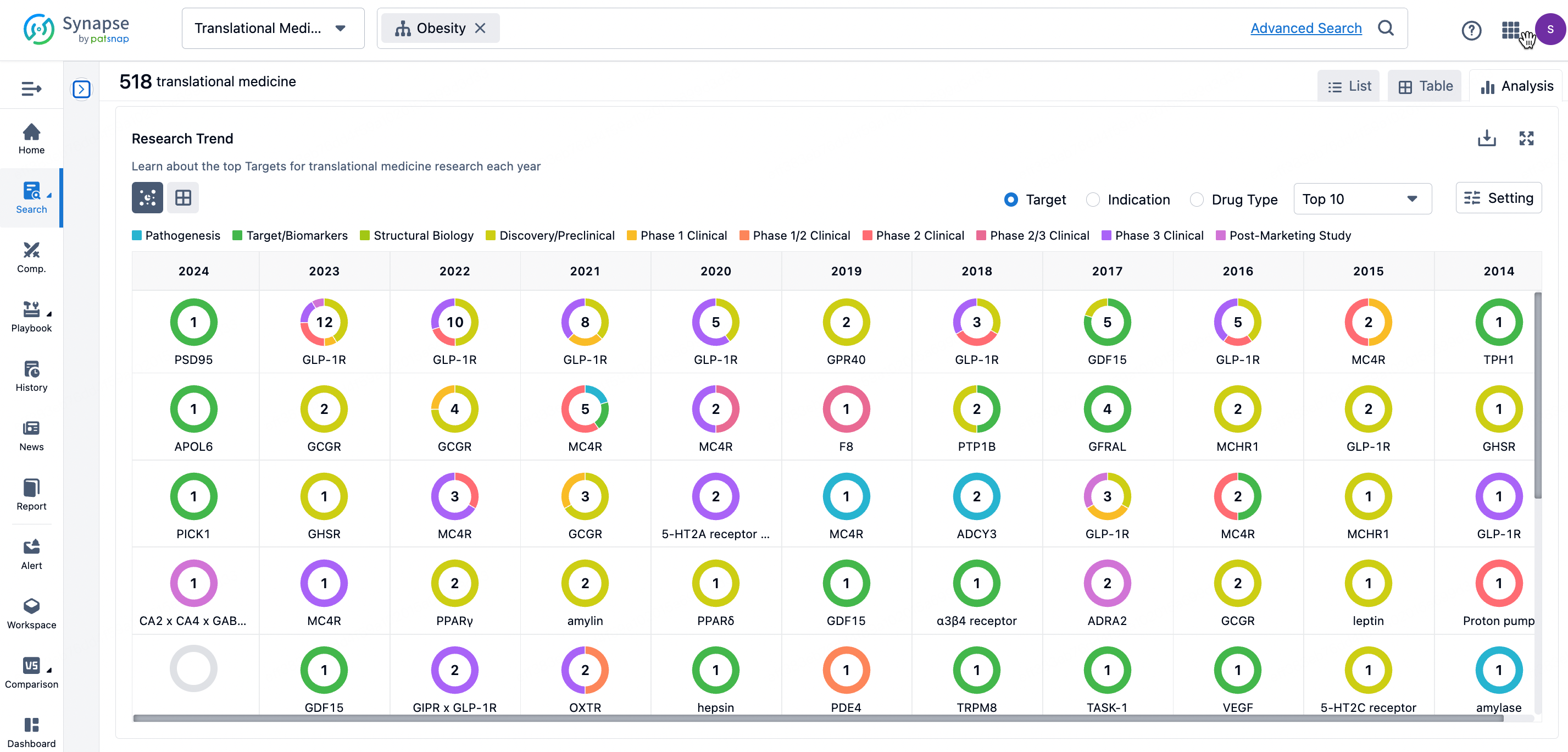
By clicking the analysis button, you can observe that GLP-1R treatment for obesity has gained significant attention over the past three years, with preclinical research still ongoing in 2023. Additionally, there are emerging potential targets, such as GDF15, among others.
Click on the image below to go directly to the Translational Medicine search interface.