2023 SITC | Junshi Biosciences announced the latest clinical results of PD-1 inhibitor Toripalimab
Recently, results from the phase 1b/2 clinical trial of Junshi Biosciences’ PD-1 inhibitor, Toripalimab, in combination with the anti-EGFR monoclonal antibody Cetuximab for the treatment of recurrent or metastatic head and neck squamous cell carcinoma (R/M-HNSCC) were presented at the poster session of the 38th Society for Immunotherapy of Cancer (SITC) annual meeting in 2023. The results showed an objective response rate (ORR) of 60% in the overall population, with patients achieving long-term tumor remission and a median duration of response (DoR) of 17.9 months.
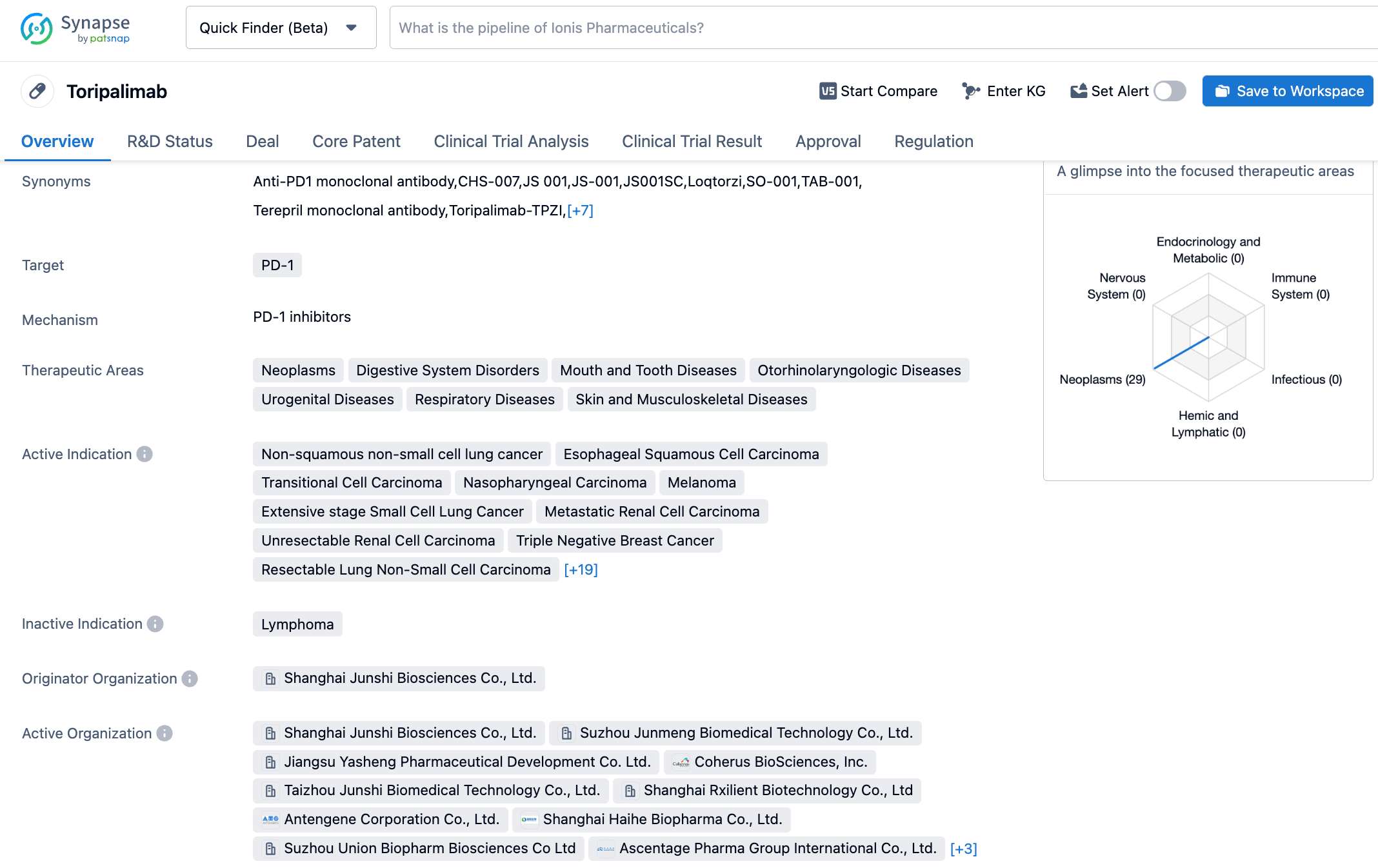
Toripalimab is independently developed by Junshi Biosciences for the treatment of various malignancies. So far, more than 40 clinical trials have been initiated by Junshi Biosciences globally (including in China, the US, Southeast Asia, and Europe) for over 15 indications. To date, this drug has been approved in China for 6 indications, which cover major cancers such as melanoma, nasopharyngeal cancer, urothelial carcinoma, esophageal squamous cell carcinoma, and non-squamous non-small cell lung cancer. In terms of internationalization, Toripalimab has been granted two breakthrough therapy designations, one fast track designation, one priority review designation, and five orphan drug designations by the US FDA in the fields of mucosal melanoma, nasopharyngeal carcinoma, soft tissue sarcoma, esophageal cancer, and small cell lung cancer.
At the SITC meeting, researchers presented detailed data from the phase 1b/2 clinical trial of Toripalimab in combination with Cetuximab for the treatment of R/M-HNSCC patients who previously failed platinum therapy. In this study, patients who showed progression of R/M-HNSCC following first-line platinum-based treatment, as well as those who progressed to R/M-HNSCC within 6 months of platinum-based neoadjuvant/adjuvant or chemoradiotherapy, were included in cohort A. All patients received combination treatment with Toripalimab and Cetuximab. The primary endpoint was the ORR assessed by an independent review committee (IRC) according to RECIST v1.1 criteria. Secondary endpoints included investigator-assessed ORR, disease control rate (DCR), DoR, progression-free survival (PFS), overall survival (OS), and safety, among others.
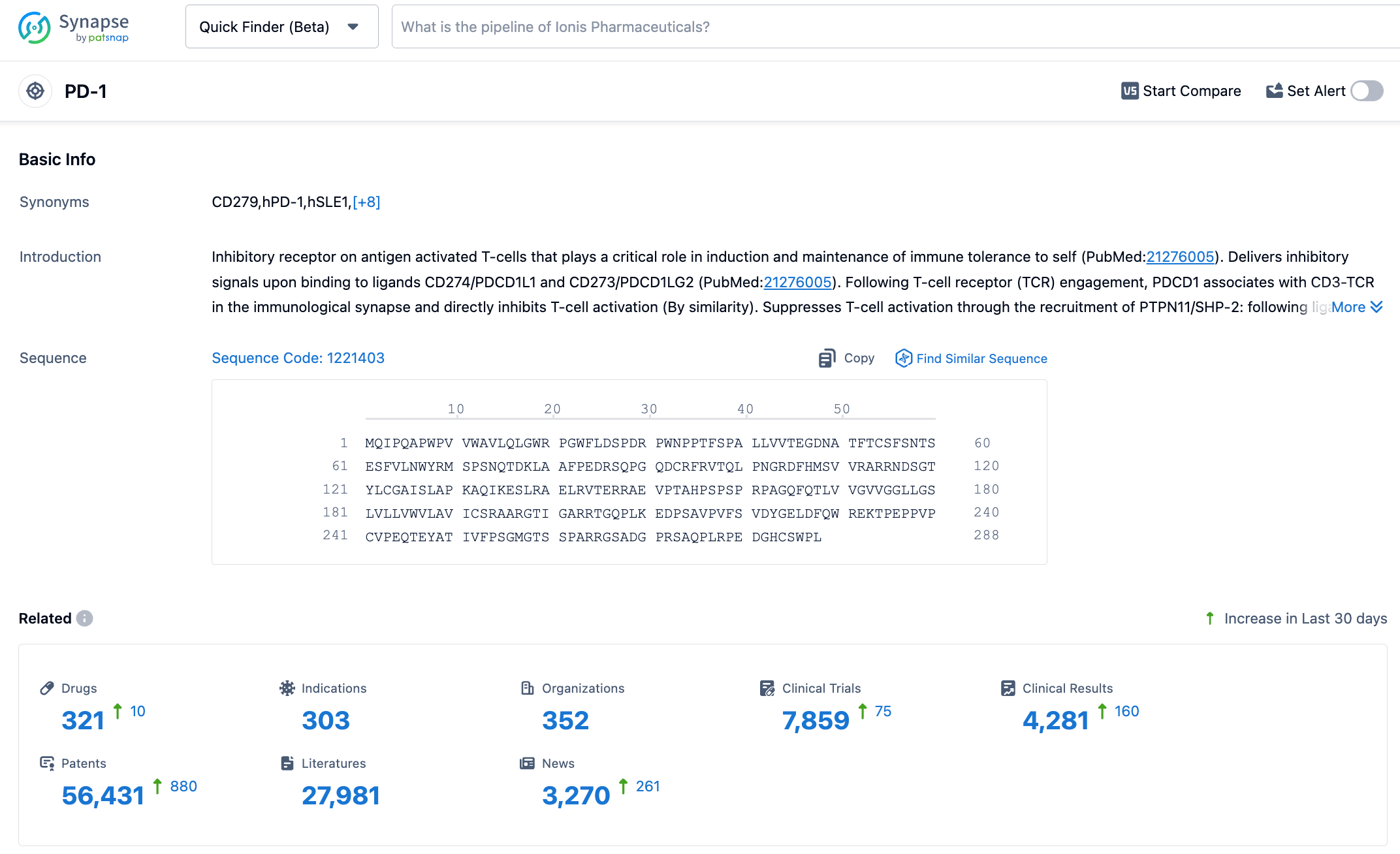
According to information disclosed by the synapse database, as of November 7, 2023, there were 320 investigational drugs targeting PD-1, with 303 indications, 351 research institutions, 7853 related clinical trials, and as many as 56431 patents. The PD-1 target is a hot area of research, and expectations are high for the future performance of Toripalimab.