8-Year Study Reveals Superior Survival with Opdivo-Yervoy vs Sunitinib for Untreated Advanced Kidney Cancer
Bristol Myers Squibb has made public its latest findings from the CheckMate -214 Phase 3 clinical study, showing that the combination treatment involving Opdivo (nivolumab) and Yervoy (ipilimumab) has sustained its effectiveness over eight years in extending the lifespan of patients who have not received prior treatment for advanced or metastatic renal cell carcinoma, when compared to sunitinib. This combination therapy has resulted in a 28% decrease in mortality risk across all risk categories as defined by the International Metastatic RCC Database Consortium.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Individuals undergoing treatment with the combined use of Opdivo and Yervoy experienced greater longevity and sustained therapeutic effects when contrasted with those receiving sunitinib treatment. Notably, this was the case for categories of patients with both intermediate and poor-risk prognoses, as well as within the totality of participants enrolled in the trial. Insights from this research are slated for dissemination via an oral session at the American Society of Clinical Oncology 2024 Genitourinary Cancers Symposium scheduled from January 25th to 27th.
"The long-term outcomes emanating from the eight-year duration of the CheckMate-214 study, which stands out as the longest follow-up interval for a Phase 3 study that evaluates combination checkpoint inhibitor therapy in the context of advanced renal cell carcinoma, are astounding," declared Nizar Tannir, M.D., F.A.C.P., faculty at the Department of Genitourinary Medical Oncology within the Division of Cancer Medicine at The University of Texas MD Anderson Cancer Center.
Dr. Tannir elaborated, stating, "The enduring advantage when juxtaposed with sunitinib is evident not just in our primary target group consisting of patients classed as intermediate and poor-risk, but also spans the wider spectrum of the study's secondary target group, which includes all participants receiving the treatment. This suggests that the nivolumab plus ipilimumab therapy may be key in helping patients with varying levels of IMDC risk attain favorable extended outcomes."
Dana Walker, M.D., M.S.C.E., Vice President and Global Program Lead for gastrointestinal and genitourinary cancers at Bristol Myers Squibb expressed, "It's an honor to witness the eight-year findings, marking the most extended survival benefit in comparison with sunitinib across any Phase 3 trial for this patient demographic. These results underscore the continuity of survival improvement offered by the front-line immunotherapeutic strategy and affirm its status as the prevailing standard of treatment for these patients."
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
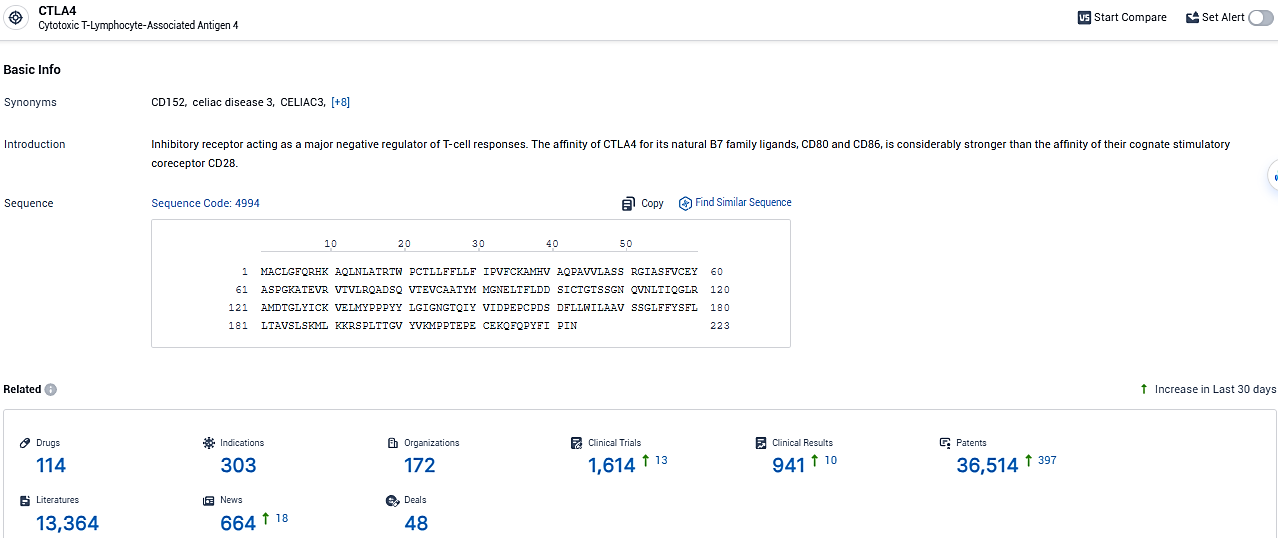
According to the data provided by the Synapse Database, As of January 29, 2024, there are 114 investigational drugs for the CTLA-4 target, including 303 indications, 172 R&D institutions involved, with related clinical trials reaching 1614, and as many as 36514 patents.
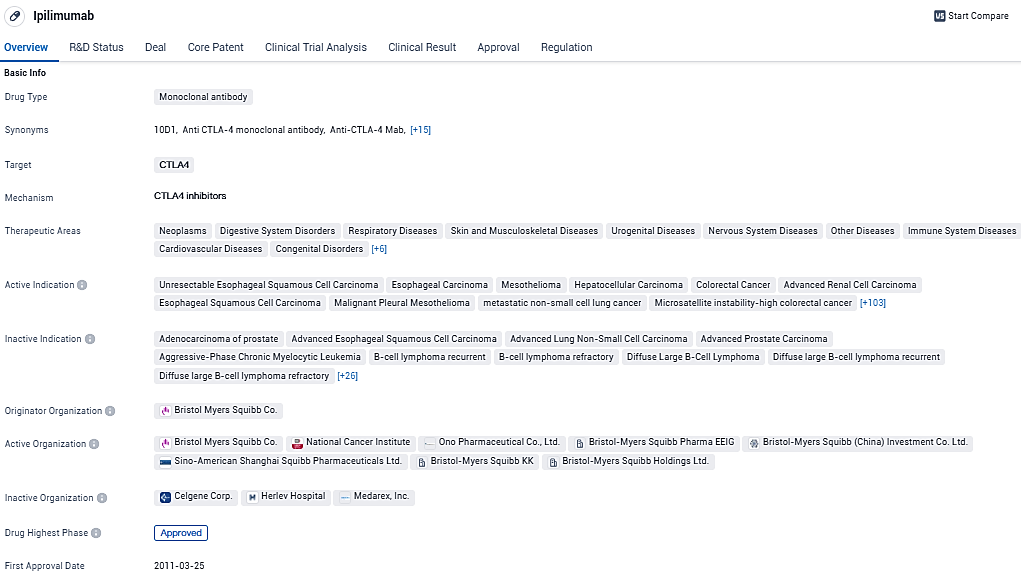
Yervoy is a recombinant, human monoclonal antibody that binds to the cytotoxic T-lymphocyte-associated antigen-4 (CTLA-4). On March 25, 2011, the U.S. Food and Drug Administration approved Yervoy 3 mg/kg monotherapy for patients with unresectable or metastatic melanoma. Yervoy is approved for unresectable or metastatic melanoma in more than 50 countries. There is a broad, ongoing development program in place for Yervoy spanning multiple tumor types.