A broad-spectrum, highly specific and high-affinity antibody: Polyclonal Antibody
A Polyclonal antibody (PAb) is a mixture of antibodies produced by different B cells in the body against the same antigen. They can recognize and bind to different antigenic sites on the same antigen. Although the advent and development of monoclonal antibodies and genetically engineered antibodies have gradually replaced polyclonal antibodies, the preparation process of polyclonal antibodies is relatively easy, and their affinity for antigens is generally higher. They are still widely used in diagnosis and treatment of diseases, as well as in experimental research. Because polyclonal antibodies have the ability to bind to different antigenic sites of a specific molecule, they are ideal reagents in diagnostic detection methods. In immunodetection, polyclonal antibodies are usually used as secondary antibodies, their role is to bind to different antigenic sites and amplify the detection signal to achieve better sensitivity.
In general, for applications that require large amounts of antibodies against the same antigenic determinant with minimal lot-to-lot variability (such as therapeutic drugs), monoclonal antibodies are a better choice. However, for common scientific research applications, polyclonal antibodies have more advantages than monoclonal antibodies. Moreover, the advantage of employing polyclonal antibodies with a higher specificity obtained from affinity purification against specific antigen in serum (reducing cross-reactive activity) will be enhanced.
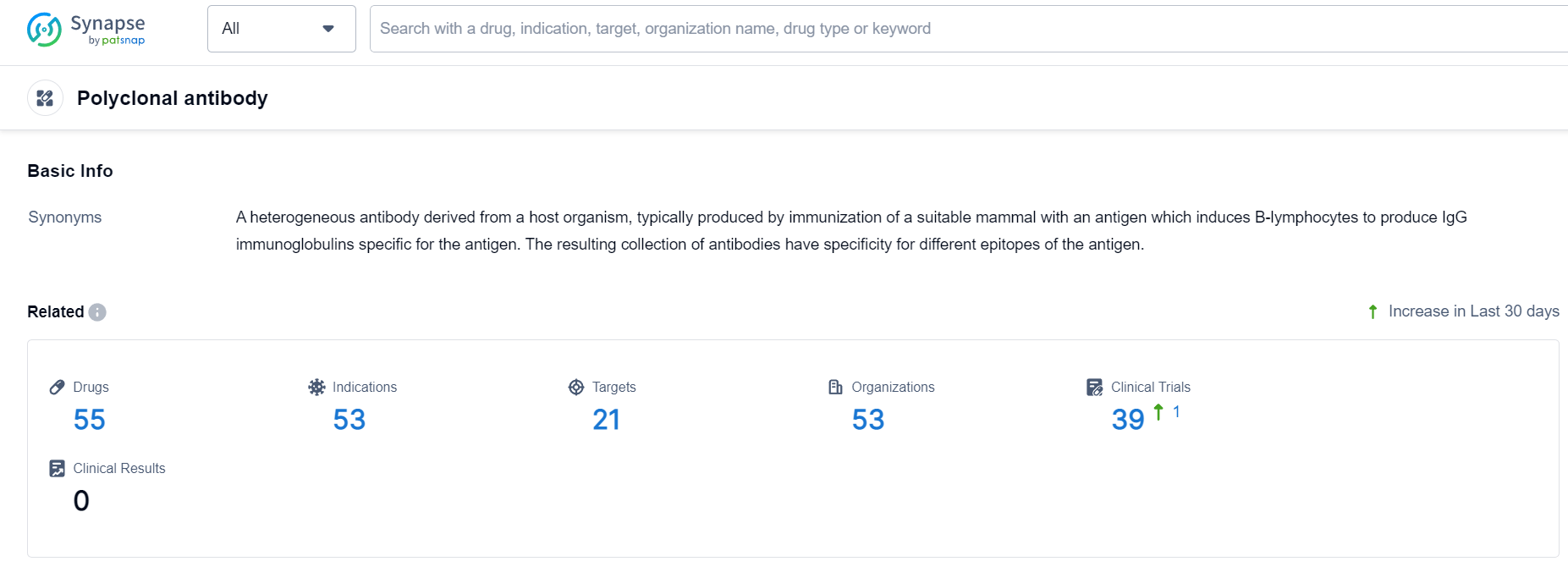
Polyclonal Antibody Competitive Landscape
According to Patsnap Synapse, as of 8 Oct 2023, there are a total of 55 Polyclonal antibody drugs worldwide, from 53 organizations, covering 21 targets, 53 indications, and conducting 39 clinical trials.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, targets, organizations, clinical trials, and clinical results related to this drug type.
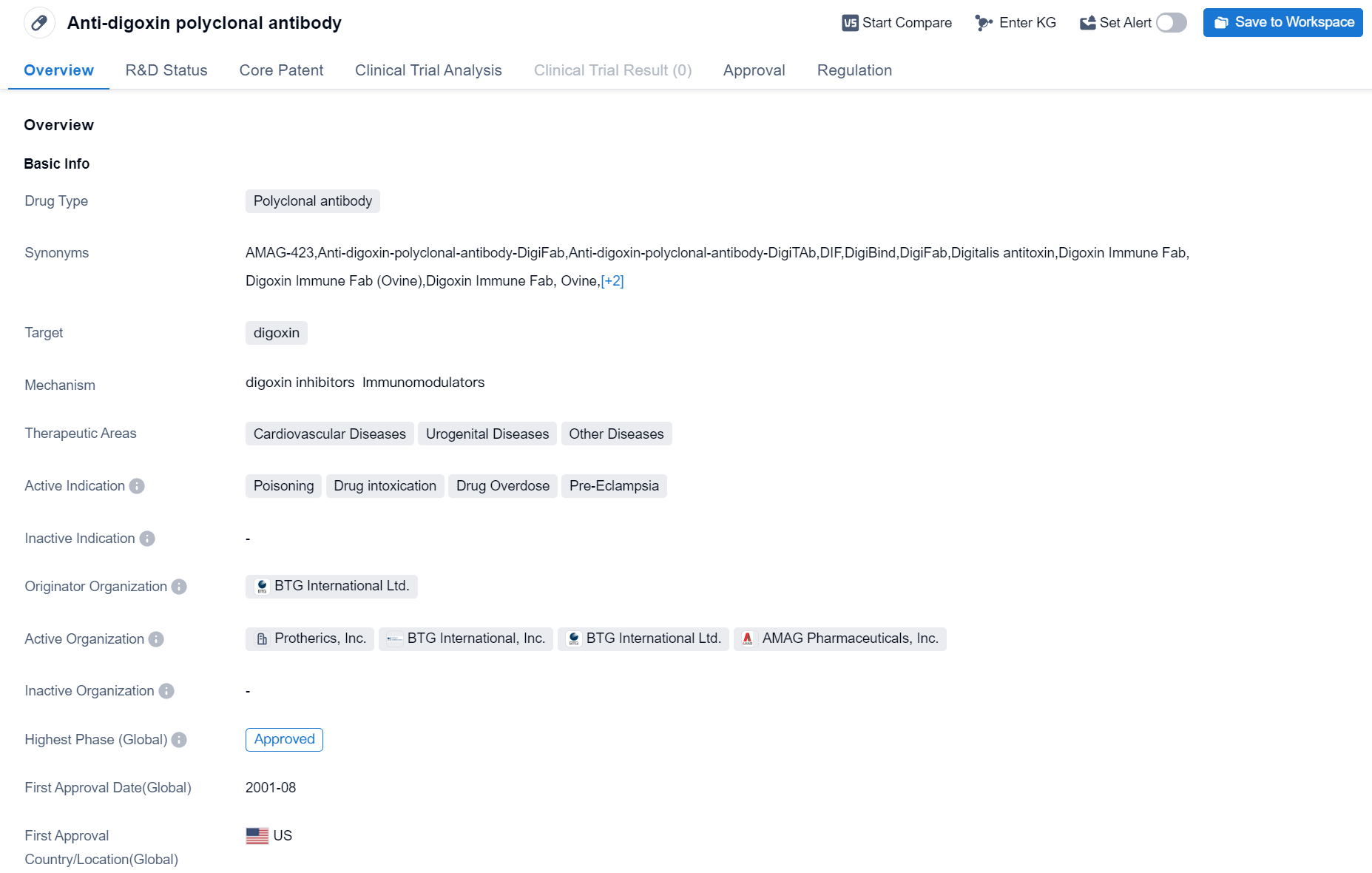
Approved Polyclonal Antibody Medicinal: Anti-digoxin polyclonal antibody
The drug Anti-digoxin polyclonal antibody is a polyclonal antibody that specifically targets digoxin. It falls under the therapeutic areas of cardiovascular diseases, urogenital diseases, and other diseases. The drug is primarily indicated for the treatment of poisoning, drug intoxication, drug overdose, and pre-eclampsia.
The originator organization of anti-digoxin polyclonal antibody is BTG International Ltd. It is important to note that the drug has reached the highest phase of development, which is approved. This indicates that it has successfully completed clinical trials and has been granted regulatory approval for use.
The first approval of anti-digoxin polyclonal antibody took place in August 2001 in the United States. It is regulated as an orphan drug, which means it is intended to treat rare diseases or conditions that affect a small number of patients.
In summary, Anti-digoxin polyclonal antibody is a polyclonal antibody drug developed by BTG International Ltd. It specifically targets digoxin and is indicated for the treatment of poisoning, drug intoxication, drug overdose, and pre-eclampsia. The drug has received regulatory approval and was first approved in the United States in August 2001. It is regulated as an orphan drug, indicating its use in treating rare diseases or conditions.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
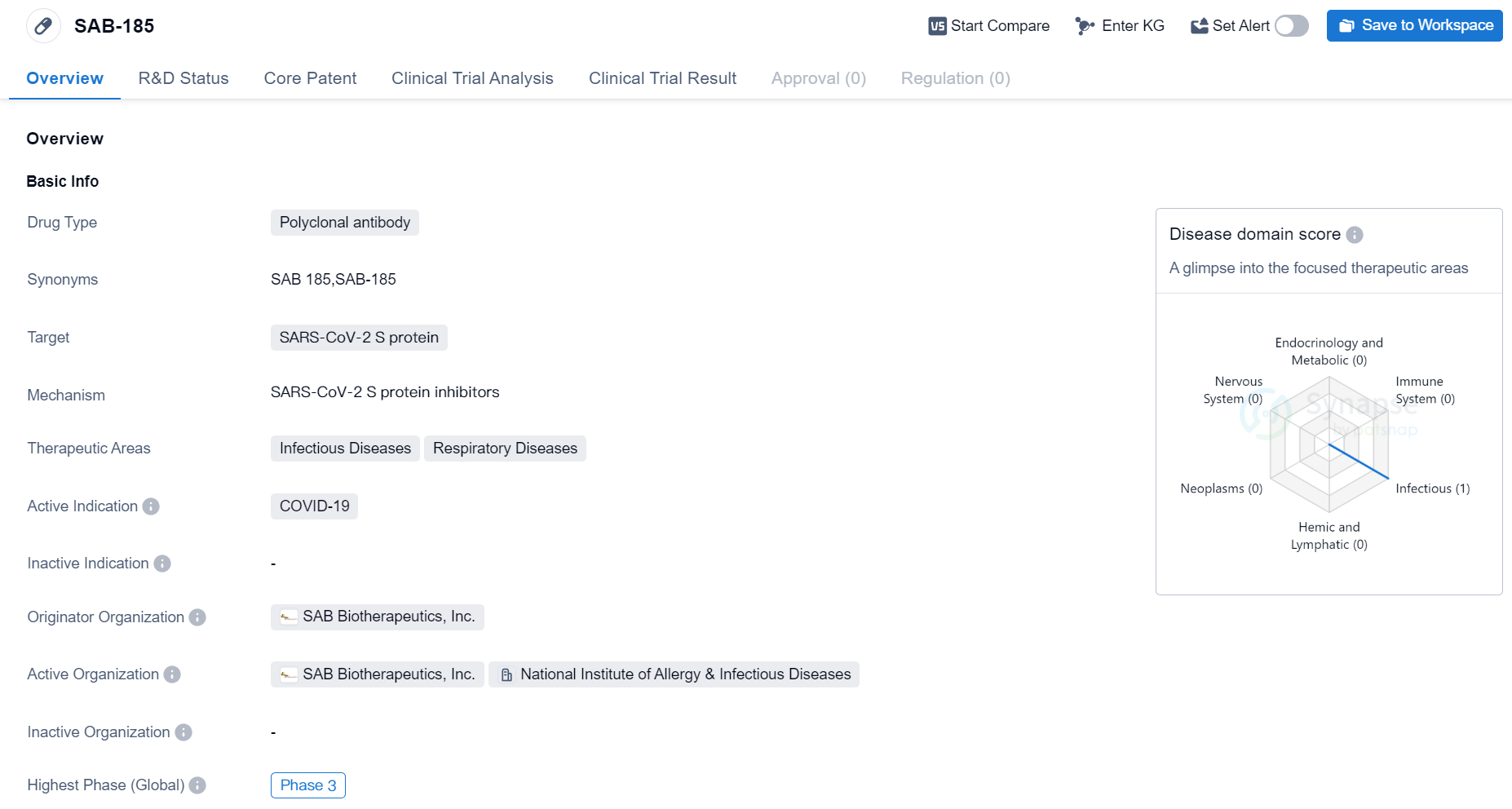
Phase III Clinical Trial of Polyclonal Antibody Medicinal: SAB-185
SAB-185 is a polyclonal antibody drug developed by SAB Biotherapeutics, Inc. It is designed to target the SARS-CoV-2 S protein, which is the main protein responsible for the entry of the virus into human cells. The drug is primarily intended for the treatment of COVID-19, a respiratory disease caused by the SARS-CoV-2 virus.
Polyclonal antibodies are a type of antibody that is derived from multiple clones of B cells, which are a type of white blood cell. These antibodies are capable of recognizing multiple epitopes on the target protein, providing a broader immune response compared to monoclonal antibodies.
The therapeutic areas of SAB-185 include infectious diseases and respiratory diseases. This suggests that the drug may have potential applications beyond COVID-19, as it could potentially be effective against other infectious respiratory diseases as well.
SAB-185 is currently in the highest phase of clinical development, which is Phase 3. This phase involves large-scale clinical trials to evaluate the safety and efficacy of the drug in a larger population. Phase 3 trials are typically conducted on thousands of patients and are crucial in determining whether a drug can be approved for commercial use.
As the originator organization, SAB Biotherapeutics, Inc. is responsible for the development and manufacturing of SAB-185.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
In summary, SAB-185 is a polyclonal antibody drug developed by SAB Biotherapeutics, Inc. It targets the SARS-CoV-2 S protein and is intended for the treatment of COVID-19. The drug is currently in Phase 3 of clinical development and has potential applications in infectious and respiratory diseases. SAB Biotherapeutics, Inc. is the originator organization behind the drug.