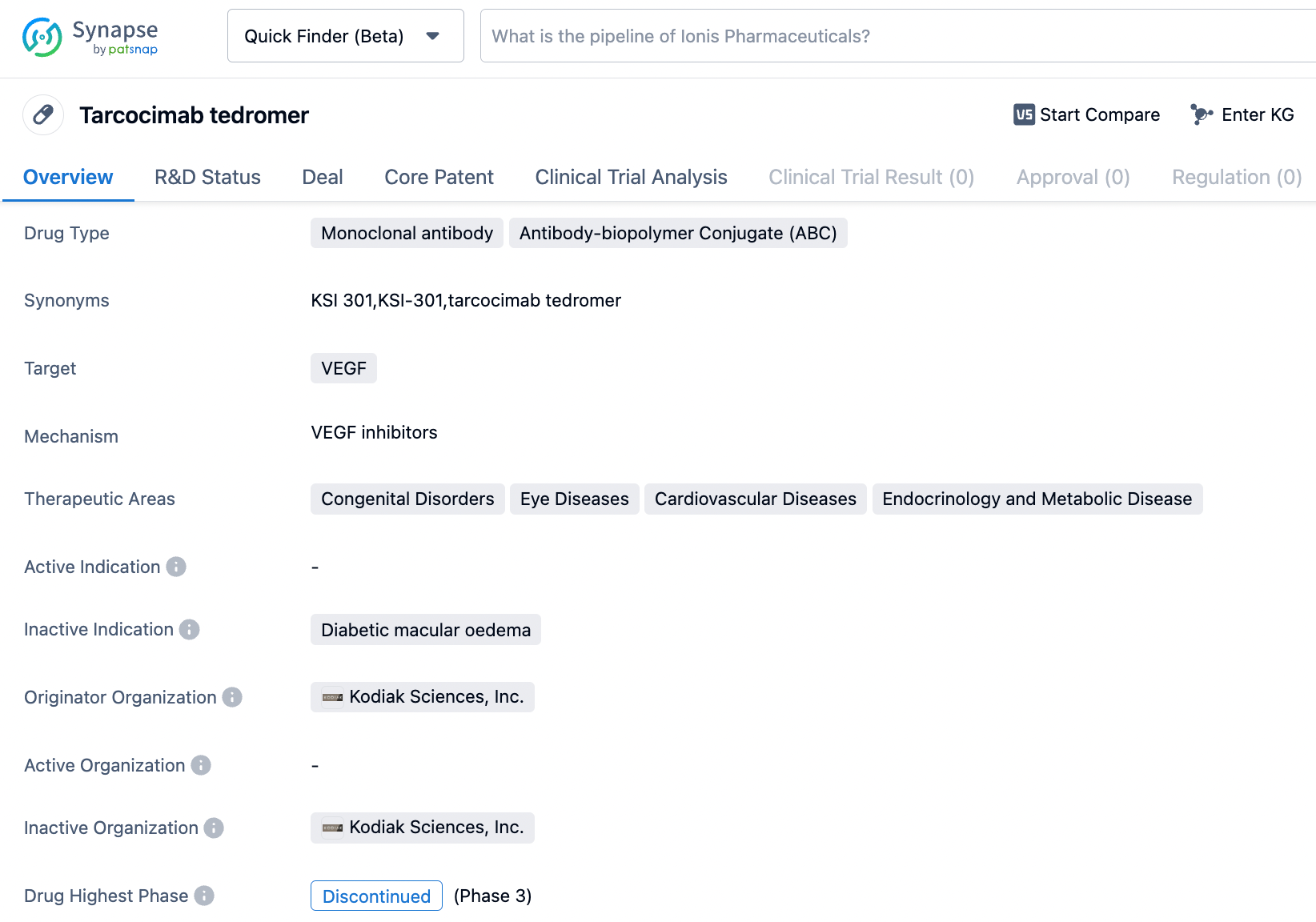
Kodiak's innovative therapy, tarcocimab tedromer, reaches primary endpoint in Phase 3 trial for the treatment of diabetic retinopathy
Recently, Kodiak Sciences announced that its Phase III GLOW study of tarcocimab tedromer, a 5 mg dose of an antibody-biocopolymer conjugate (ABC) therapy for the treatment of moderate to severe non-proliferative diabetic retinopathy (NPDR) patients, achieved its primary endpoint at one year. Following treatment every six months, improvements and stability in the vision and retinal anatomy of NPDR patients were observed.
Tarcocimab tedromer (KSI-301) is a long-acting anti-vascular endothelial growth factor (VEGF) therapy developed by Kodiak based on its proprietary ABC platform. By coupling VEGF antibodies to a highly hydrophobic phosphocholine copolymer, it specifically binds to VEGF to downregulate VEGF levels and significantly prolongs the half-life of the VEGF antibody in the vitreous, thereby achieving longer effectiveness and reducing the frequency of administration. In vitro test results have shown that KSI-301 compared to the Maximal Inhibition of ranibizumab and abicipar had improved inhibition of HRMVEC cell proliferation.
Analyses indicated the GLOW study achieved its primary endpoint - the ratio of patients whose Diabetic Retinopathy Severity Score (DRSS) improved by at least two levels. In patients treated with tarcocimab, 41.1% of evaluable patients improved by at least 2 levels, compared to just 1.4% in the sham treatment control group (P<0.0001), a 29-fold increase in response rate. Vision and retinal anatomy improved and remained stable even after extending the dosing interval. The GLOW study also met all key secondary endpoints, including significantly greater reductions in the proportion of patients with vision-threatening complications (such as diabetic macular edema and proliferative diabetic retinopathy) compared to the control group. This equated to an 89% risk reduction, or 21.0% compared to 2.3% (P<0.0001). The risk of developing diabetic macular edema was reduced by 95% compared to the control group, with incidence rates of 13.7% in the control group and 0.7% in the tarcocimab group. Similar incidence rates of serious ocular adverse events and intra-ocular inflammation were observed in both groups.
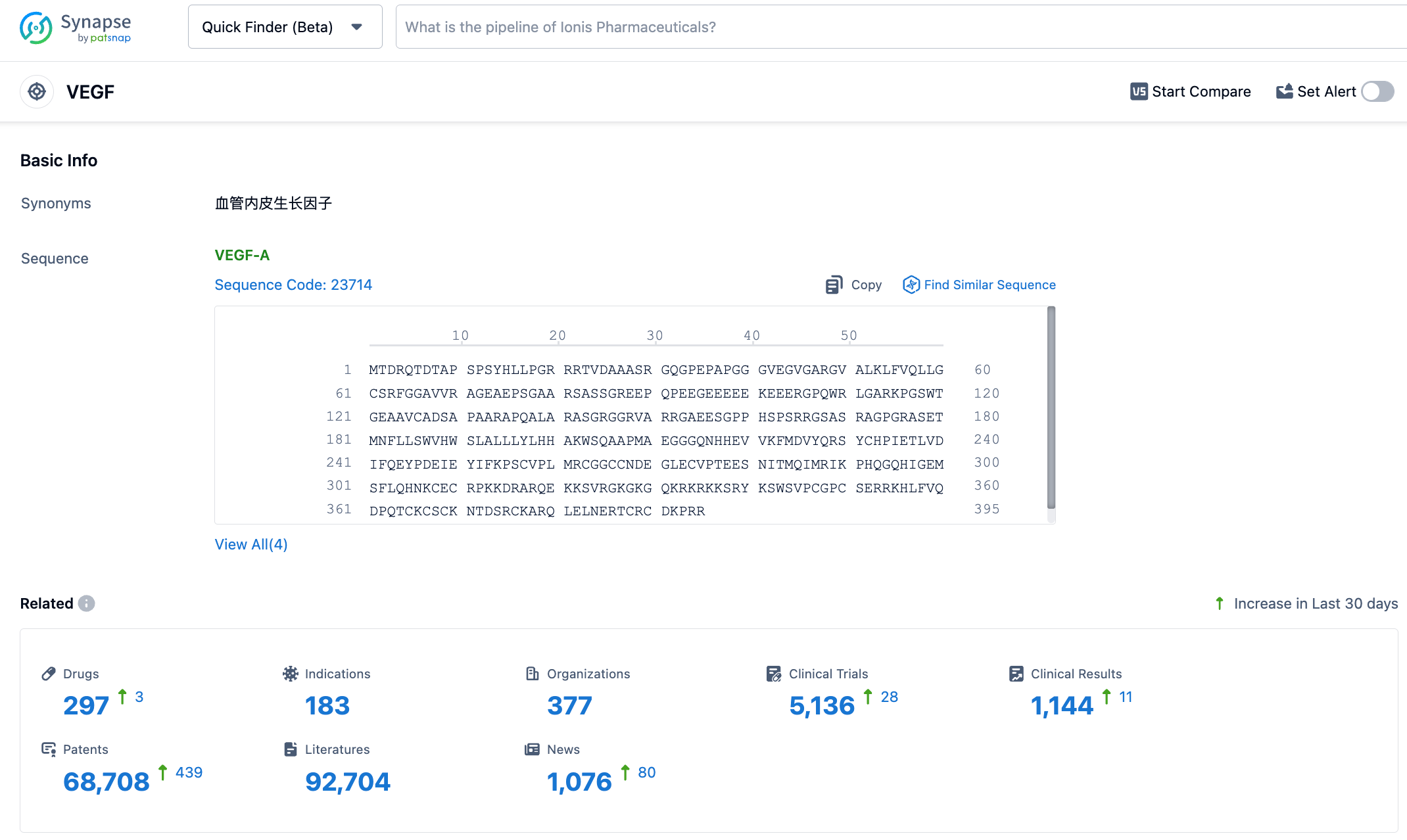
According to the information disclosed by the Synapse Database, as of November 9, 2023, there are 297 drugs under research targeted at VEGF, applied for 183 indications, by 377 institutions, involved in 5130 clinical trials, and filed for as many as 68663 patents. On July 24, 2023, Kodiak Sciences released the latest data from three Phase III studies of tarcocimab tedromer for the treatment of AMD and DME, none of which achieved the primary endpoint, resulting in a significant fall in share price. The recent achievement of the one-year primary endpoint in the phase III GLOW study of tarcocimab tedromer for the treatment of moderate to severe NPDR patients could see a turnaround. The results are eagerly awaited.