Adagene Showcases Findings Exhibiting Top-of-the-Line Therapeutic Index for Concealed Anti-CTLA-4 SAFEbody® ADG126 at 2023 SITC Event
At the 38th Annual Society for Immunotherapy of Cancer Meeting in San Diego, Adagene Inc., a firm that is revolutionizing the creation and advancement of new antibody-related therapies, showcased fresh information about its covered anti-CTLA-4 SAFEbody ADG126.
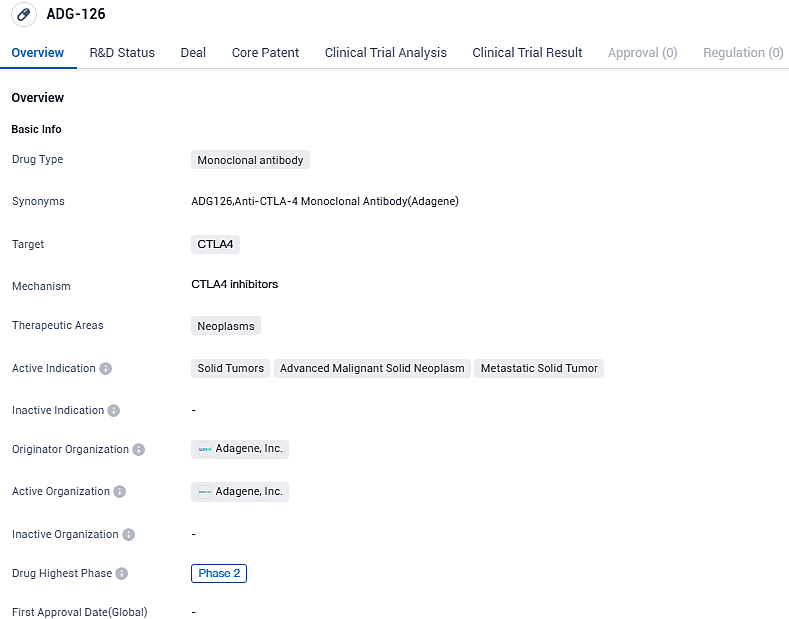
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The analyzed information, which unifies clinical outcomes with physiologically based pharmacokinetic and quantitative systems pharmacology modeling, revealed that Adagene's primary SAFEbody contender, ADG126, is proficient at targeting CTLA-4 within the tumor microenvironment.
This culminated in a roughly 30-fold anticipated pharmacokinetic disparity at a dosage of 10 mg/kg every three weeks in the TME, implying a broader therapeutic index in comparison to ipilimumab at 1 mg/kg every six weeks, when teamed up with anti-PD-1 treatments.
The enhanced therapeutic index of ADG126 fosters larger, more regular, and recurring doses of ADG126 in synergy with anti-PD-1, leading to a substantial boost in CTLA-4 interaction by the activated ADG126 at steady-state in tumors versus circulating blood.
Scrutinies further revealed that the ideal dose of ADG126 at 10 mg/kg every three weeks combined with pembrolizumab culminates in a dose-dependent effectiveness profile, without significantly heightening treatment-related adverse occurrences.
Significantly, a clinical instance provided for the first time from a present dose expansion group in advanced/metastatic MSS CRC* patients excluding liver metastases showed that ADG126 at 10 mg/kg every three weeks along with pembrolizumab led to a confirmed partial response after four cycles. The patient had previously undergone two lines of treatment and had manageable Grade 3 treatment-related adverse events that were consistent with known side effects from immunotherapy.
ADG126 SAFEbody is the most progressed clinical phase anti-CTLA-4 candidate incorporating masking technology and Treg reduction for superior safety and efficacy profiles. A phase 2 dose expansion cohort is continuing to assess ADG126 combined with pembrolizumab in patients with MSS CRC devoid of liver metastases.
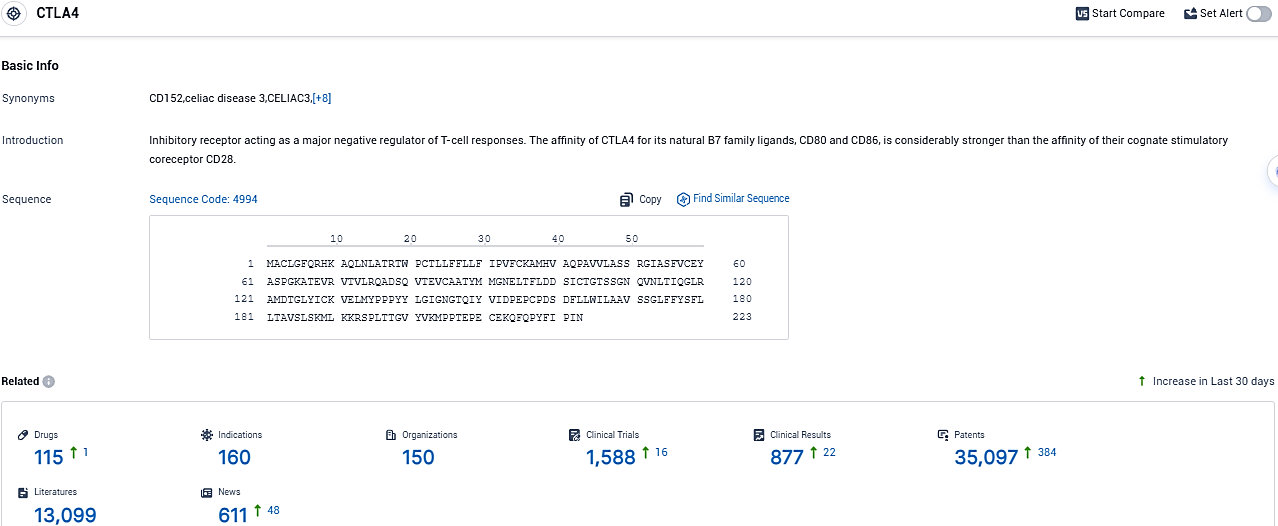
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of November 11, 2023, there are 115 investigational drugs for the CTLA4 target, including 160 indications, 150 R&D institutions involved, with related clinical trials reaching 1588, and as many as 35097 patents.
ADG-126 is indicated for the treatment of solid tumors, including advanced malignant solid neoplasms and metastatic solid tumors. The drug has reached Phase 2 globally, indicating promising results in earlier stages of clinical trials, while in China, it is in Phase 1. ADG-126's development highlights the potential of monoclonal antibodies in the field of biomedicine for targeted cancer therapies.