A Comprehensive Review of peginterferon beta-1a's R&D Innovations and Drug Target Mechanism
Peginterferon beta-1a's R&D Progress
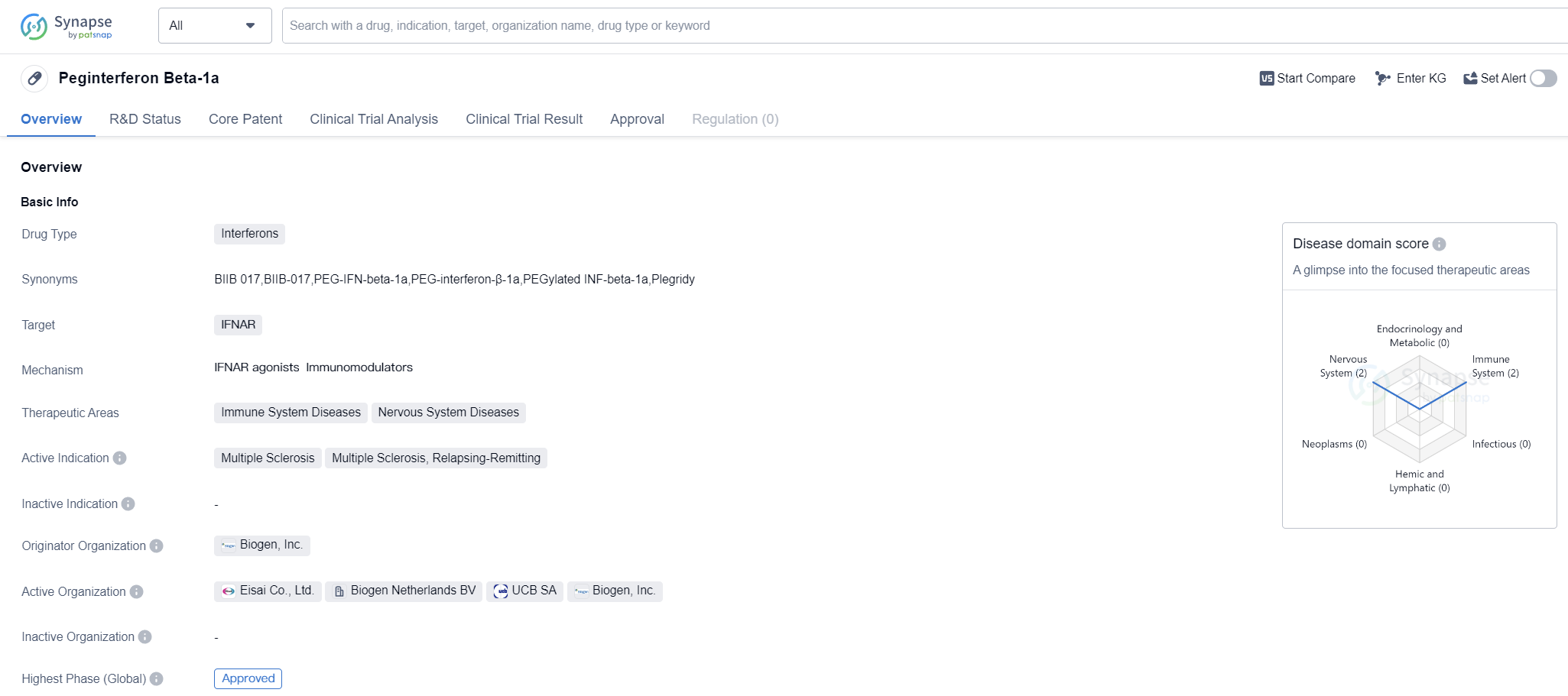
Peginterferon Beta-1a is a drug classified as an interferon and is primarily used to target the IFNAR receptor. It is indicated for the treatment of immune system diseases and nervous system diseases, specifically multiple sclerosis (MS) and relapsing-remitting multiple sclerosis (RRMS).
The drug was first approved in the European Union in July 2014, making it available for use in that region. It is important to note that the drug has reached the highest phase of development, which means it has successfully completed clinical trials and has been approved for commercial use.
Peginterferon Beta-1a is developed by Biogen, Inc., a well-known organization in the pharmaceutical industry. Biogen is the originator of this drug, indicating that they were responsible for its initial development and subsequent approval.
Multiple sclerosis is a chronic autoimmune disease that affects the central nervous system, causing inflammation and damage to the protective covering of nerve fibers. RRMS is the most common form of MS, characterized by periods of relapse and remission.
Interferons are a type of protein that play a crucial role in regulating the immune system. By targeting the IFNAR receptor, Peginterferon Beta-1a helps modulate the immune response, reducing inflammation and potentially slowing down the progression of MS.
The approval of Peginterferon Beta-1a in the European Union in 2014 marked an important milestone in the treatment of MS and RRMS. It provided healthcare professionals and patients with a new therapeutic option to manage these conditions.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for peginterferon beta-1a: IFNAR agonists Immunomodulators
IFNAR agonists are a type of immunomodulators. Immunomodulators are substances that can modify or regulate the immune system's response. In the case of IFNAR agonists, they specifically target and activate the IFNAR (interferon-alpha/beta receptor) present on immune cells.
The IFNAR agonists stimulate the immune system by binding to the IFNAR, leading to the activation of various signaling pathways. This activation triggers the production and release of interferons, which are proteins that play a crucial role in regulating immune responses. Interferons help in enhancing the immune system's ability to fight off infections, tumors, and other diseases.
By acting as immunomodulators, IFNAR agonists can be used in the treatment of various conditions, including viral infections, autoimmune disorders, and certain types of cancers. They work by boosting the immune response and promoting the body's natural defense mechanisms.
It's important to note that the explanation provided here is from a biomedical perspective, focusing on the role of IFNAR agonists as immunomodulators.
Drug Target R&D Trends for peginterferon beta-1a
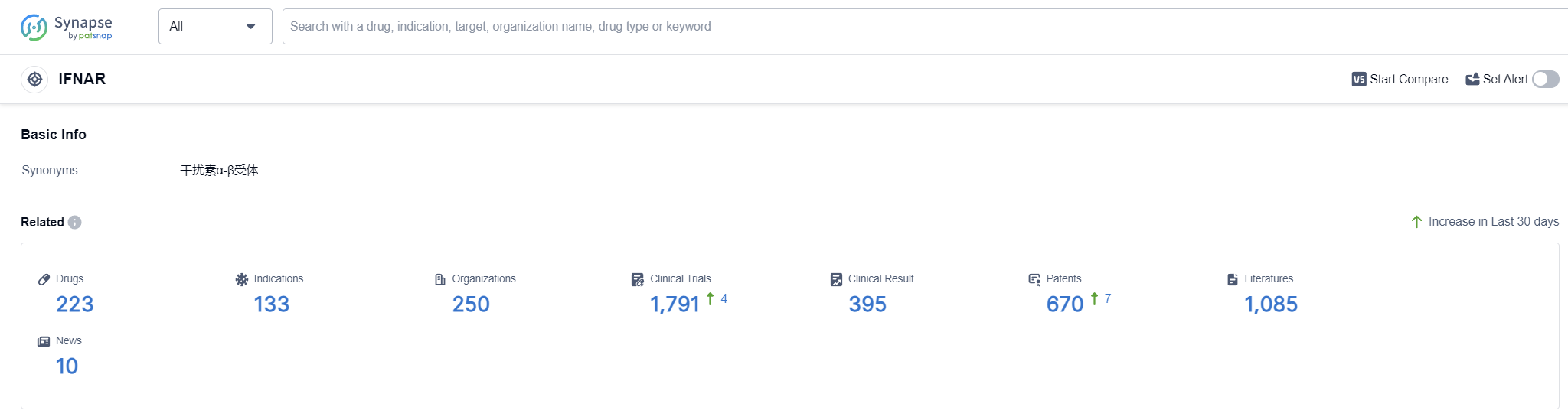
IFNAR, or interferon alpha/beta receptor, plays a crucial role in the human body's immune response. It is a receptor protein found on the surface of cells and is responsible for binding to interferon-alpha and interferon-beta, which are important signaling molecules involved in antiviral defense. When IFNAR binds to these interferons, it triggers a cascade of cellular responses that help to inhibit viral replication, activate immune cells, and enhance the body's ability to fight off infections. Understanding the role of IFNAR is essential in developing therapies that target the immune system and combat viral diseases.
According to Patsnap Synapse, as of 12 Sep 2023, there are a total of 223 IFNAR drugs worldwide, from 250 organizations, covering 133 indications, and conducting 1791 clinical trials.
The analysis of the target IFNAR reveals a competitive landscape with multiple companies focusing on its development. Danaher Corp., Merck & Co., Inc., Roche Holding AG, and Beijing Kawin Technology Share-Holding Co., Ltd. are the companies growing fastest under this target. The most common indications for approved drugs include Hepatitis C, Hepatitis B, and Hairy Cell Leukemia. Interferons and biosimilars are the drug types progressing most rapidly, indicating intense competition. China is the country developing fastest under the target IFNAR, followed by the United States, Japan, and the European Union.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
In summary, Peginterferon Beta-1a is an interferon-based drug developed by Biogen, Inc. It is indicated for the treatment of immune system diseases and nervous system diseases, specifically multiple sclerosis and relapsing-remitting multiple sclerosis. The drug received its first approval in the European Union in 2014 and has reached the highest phase of development. Its mechanism of action involves targeting the IFNAR receptor to modulate the immune response.