A Comprehensive Review of Somatostatin's R&D Innovations
Somatostatin's R&D Progress
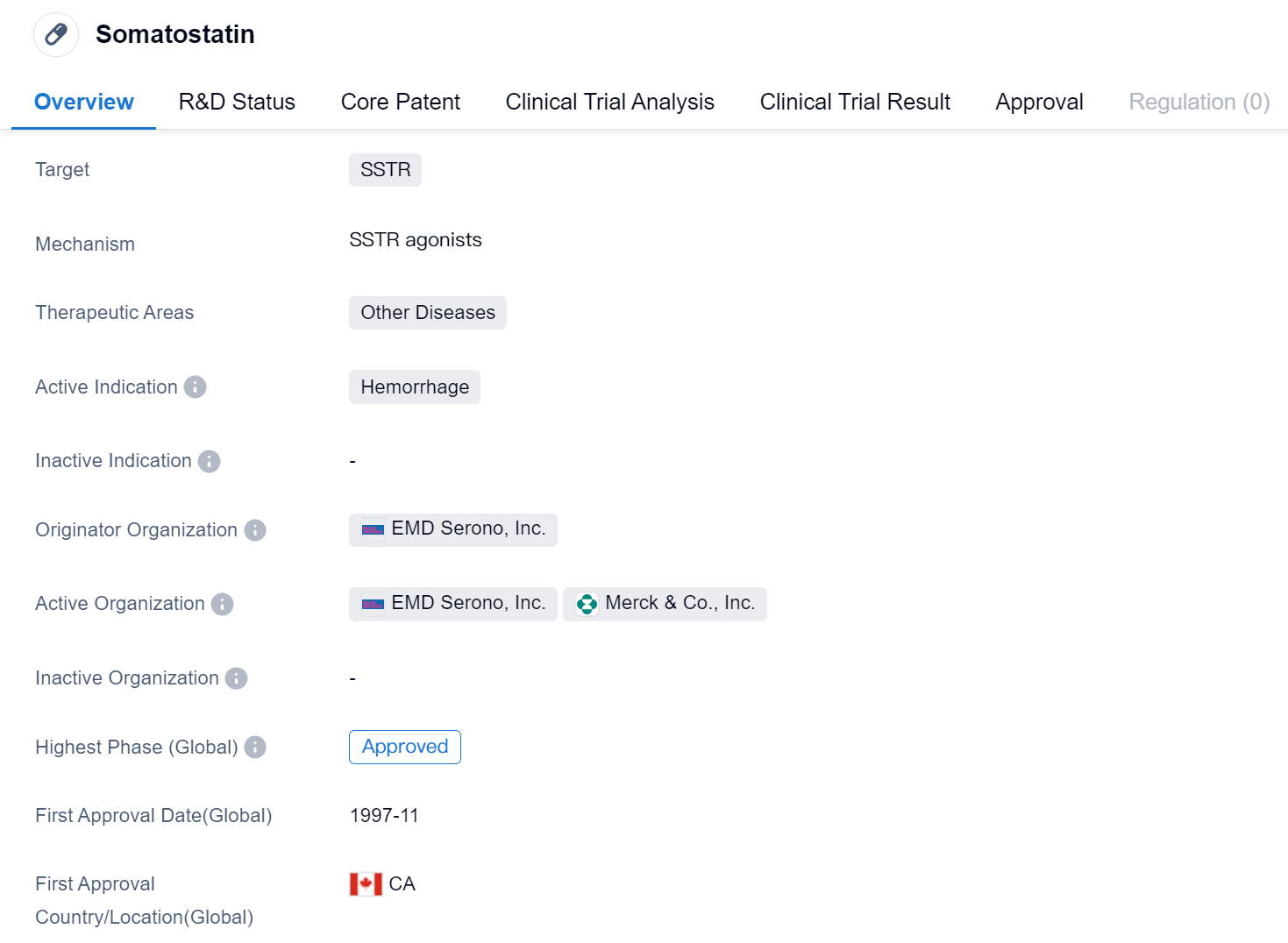
Somatostatin is a synthetic peptide drug that targets somatostatin receptors (SSTR) and is primarily used in the treatment of hemorrhage. It falls under the therapeutic area of other diseases. The drug was first approved in Canada in November 1997 and has since received approval in other countries globally.
The originator organization of Somatostatin is EMD Serono, Inc., a pharmaceutical company specializing in the development and commercialization of innovative therapies. As the highest phase of development for Somatostatin is approved, it indicates that the drug has met the regulatory requirements and has been granted market authorization.
Being a synthetic peptide, Somatostatin is designed to mimic the natural hormone somatostatin, which plays a crucial role in regulating various physiological processes in the body. By targeting SSTR, the drug aims to modulate the activity of somatostatin receptors, thereby exerting its therapeutic effects in the treatment of hemorrhage.
The drug's approval in 1997 suggests that it has been available for clinical use for over two decades. Therefore, Somatostatin has established itself as a reliable therapeutic option for patients suffering from hemorrhage.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Somatostatin: SSTR agonists
SSTR agonists refer to substances or drugs that activate or stimulate somatostatin receptors (SSTRs). Somatostatin is a hormone that regulates various physiological processes in the body, including the inhibition of the release of other hormones. SSTR agonists specifically bind to and activate the somatostatin receptors, leading to a cascade of cellular events.
From a biomedical perspective, SSTR agonists are of particular interest in the field of biomedicine because they have potential therapeutic applications. By activating SSTRs, these agonists can modulate hormone secretion, inhibit tumor growth, and regulate various physiological functions. They are commonly used in the diagnosis and treatment of neuroendocrine tumors, such as carcinoid tumors and pituitary adenomas.
SSTR agonists can be administered through different routes, including injection, infusion, or oral administration, depending on the specific drug formulation. They may have varying affinities for different subtypes of somatostatin receptors, such as SSTR1, SSTR2, SSTR3, SSTR4, and SSTR5. The specific agonist chosen for a particular application depends on the targeted receptor subtype and the desired therapeutic outcome.
Overall, SSTR agonists play a crucial role in biomedical research and clinical practice, offering potential benefits in the management of various diseases and conditions related to hormone dysregulation and tumor growth.
Drug Target R&D Trends for Somatostatin
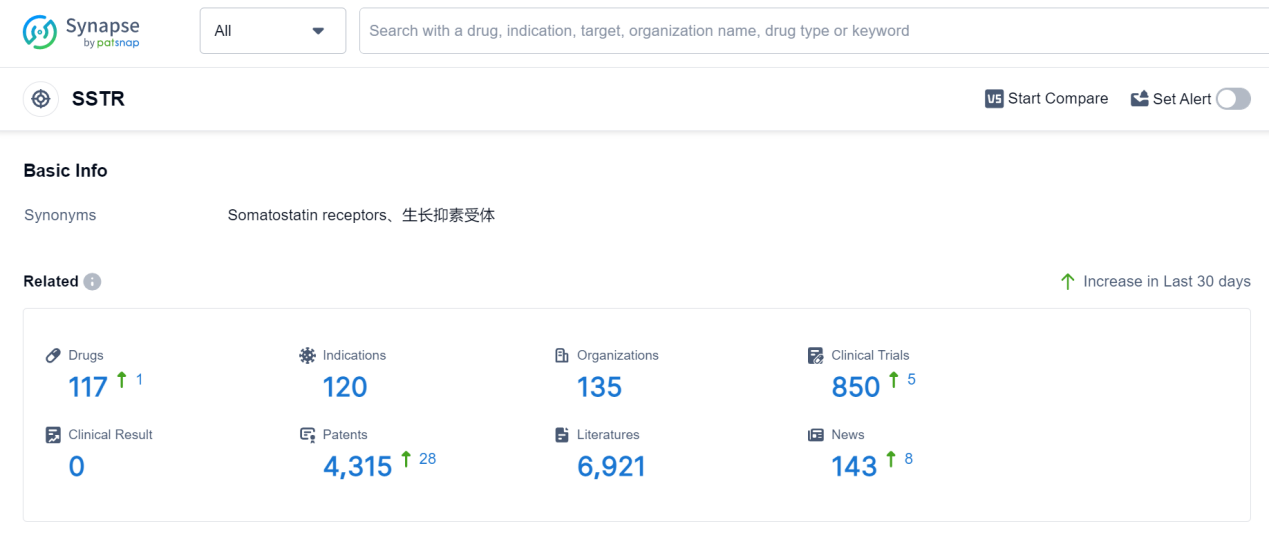
SSTR, or somatostatin receptors, play a crucial role in the human body. These receptors are found in various tissues and organs, including the brain, pancreas, and gastrointestinal tract. SSTRs are responsible for binding to somatostatin, a hormone that regulates the release of other hormones and neurotransmitters. By binding to SSTRs, somatostatin can inhibit the secretion of growth hormone, insulin, glucagon, and other substances. This regulatory function helps maintain hormonal balance and control various physiological processes, such as growth, metabolism, and digestion. Understanding the role of SSTRs is essential in developing targeted therapies for conditions like acromegaly, neuroendocrine tumors, and other disorders related to hormone dysregulation.
According to Patsnap Synapse, as of 7 Sep 2023, there are a total of 117 SSTR drugs worldwide, from 135 organizations, covering 120 indications, and conducting 850 clinical trials.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
In summary, Somatostatin is a synthetic peptide drug developed by EMD Serono, Inc. It targets SSTR and is primarily used in the treatment of hemorrhage. The drug has received approval in multiple countries, including Canada and China. With its long history of clinical use, Somatostatin has established itself as a reliable therapeutic option for patients suffering from hemorrhage.