Deep Scientific Insights on Ranolazine's R&D Progress,Drug Target
Ranolazine's R&D Progress
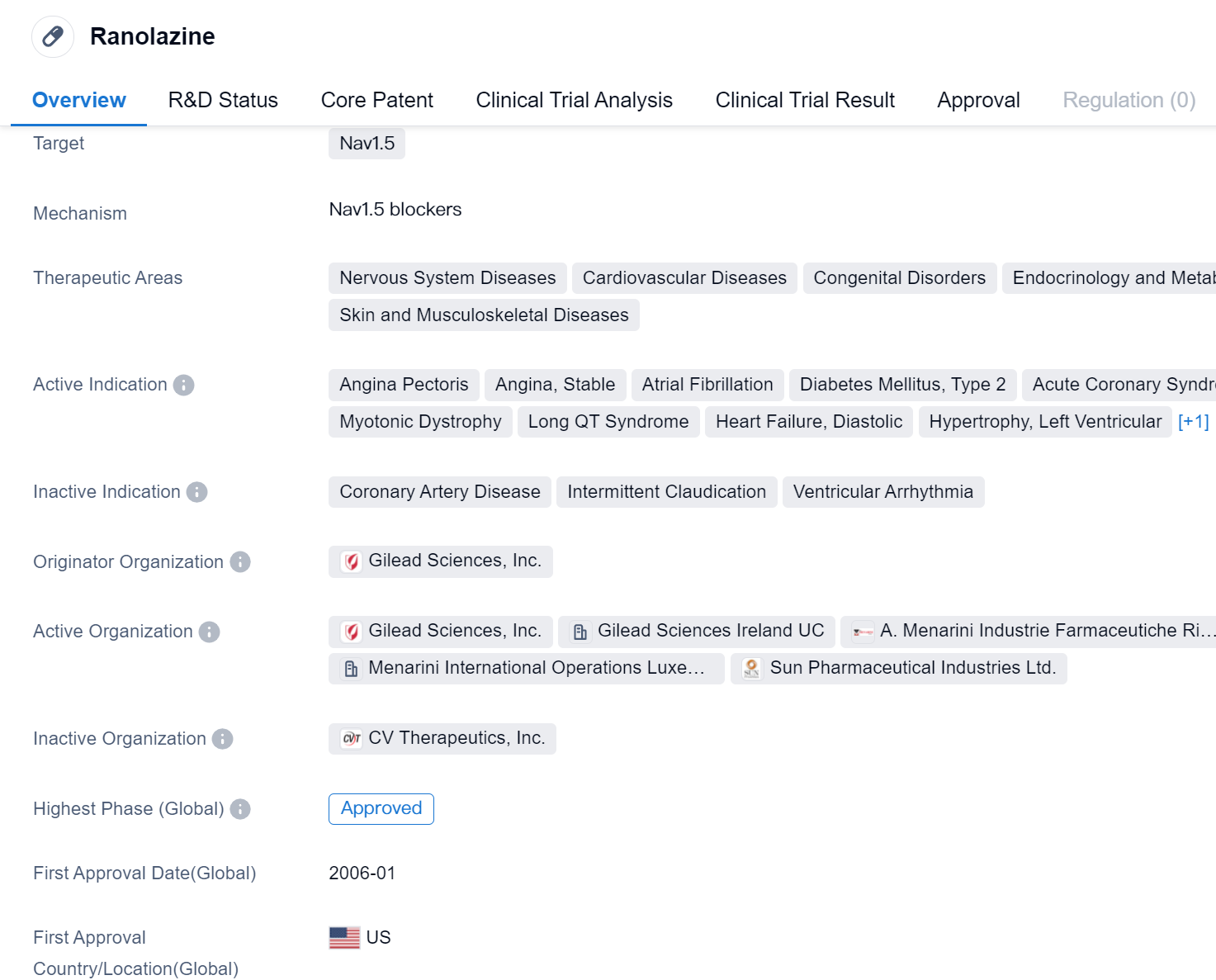
Ranolazine is a small molecule drug that primarily targets Nav1.5, a protein involved in the regulation of sodium channels in the body. It has been approved for use in various therapeutic areas, including Nervous System Diseases, Cardiovascular Diseases, Congenital Disorders, Endocrinology and Metabolic Disease, as well as Skin and Musculoskeletal Diseases.
The drug has shown efficacy in treating several conditions, including Angina Pectoris, Angina (Stable), Atrial Fibrillation, Diabetes Mellitus (Type 2), Acute Coronary Syndrome, Amyotrophic Lateral Sclerosis, Myotonic Dystrophy, Long QT Syndrome, Heart Failure (Diastolic), Hypertrophy (Left Ventricular), and Aortic Valve Stenosis.
Ranolazine was developed by Gilead Sciences, Inc., a renowned pharmaceutical company. It received its first approval in the United States in January 2006, making it available for use in patients in that country.
The approval of Ranolazine marks its highest phase of development, indicating that it has successfully completed clinical trials and met the necessary regulatory requirements for market authorization. This suggests that the drug has demonstrated safety and efficacy in treating the indicated conditions.
Ranolazine's approval in multiple therapeutic areas highlights its potential to address a wide range of medical conditions. Its targeting of Nav1.5, a protein involved in sodium channel regulation, suggests that it may have a mechanism of action that is beneficial in various disease pathways.
The drug's approval for the treatment of Angina Pectoris, a condition characterized by chest pain due to reduced blood flow to the heart, is particularly significant. It provides an additional treatment option for patients suffering from this common cardiovascular condition.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Ranolazine: Nav1.5 blockers
Nav1.5 blockers are a type of medication or compound that specifically target and inhibit the Nav1.5 sodium channels. These sodium channels, also known as voltage-gated sodium channels, are found in various tissues throughout the body, including the heart. They play a crucial role in the generation and conduction of electrical signals in excitable cells.
From a biomedical perspective, Nav1.5 blockers are primarily used in the context of cardiac electrophysiology. By blocking the Nav1.5 sodium channels, these medications can modify the electrical activity of the heart. This can be beneficial in certain conditions where abnormal electrical signals or arrhythmias are present.
Nav1.5 blockers are commonly used in the treatment of cardiac arrhythmias, particularly those associated with atrial fibrillation. By inhibiting the Nav1.5 channels, these blockers can help regulate the electrical impulses in the heart, restoring normal rhythm and reducing the risk of complications.
It's important to note that Nav1.5 blockers are a specific type of medication that target a specific ion channel. There are various types of Nav1.5 blockers available, each with its own mechanism of action and efficacy. These medications are typically prescribed by healthcare professionals and should be used under their guidance and supervision.
Drug Target R&D Trends for Ranolazine
Nav1.5, also known as the voltage-gated sodium channel Nav1.5, plays a crucial role in the human body. It is primarily found in cardiac cells and is responsible for the initiation and propagation of electrical signals that regulate the heart's rhythm. Nav1.5 facilitates the rapid influx of sodium ions into cardiac cells, enabling the depolarization necessary for proper heart function. Mutations in the SCN5A gene, which encodes Nav1.5, can lead to various cardiac arrhythmias and disorders, highlighting the significance of this channel in maintaining a healthy heart rhythm. Understanding Nav1.5's role is essential for developing targeted therapies to treat cardiac conditions and improve patient outcomes.
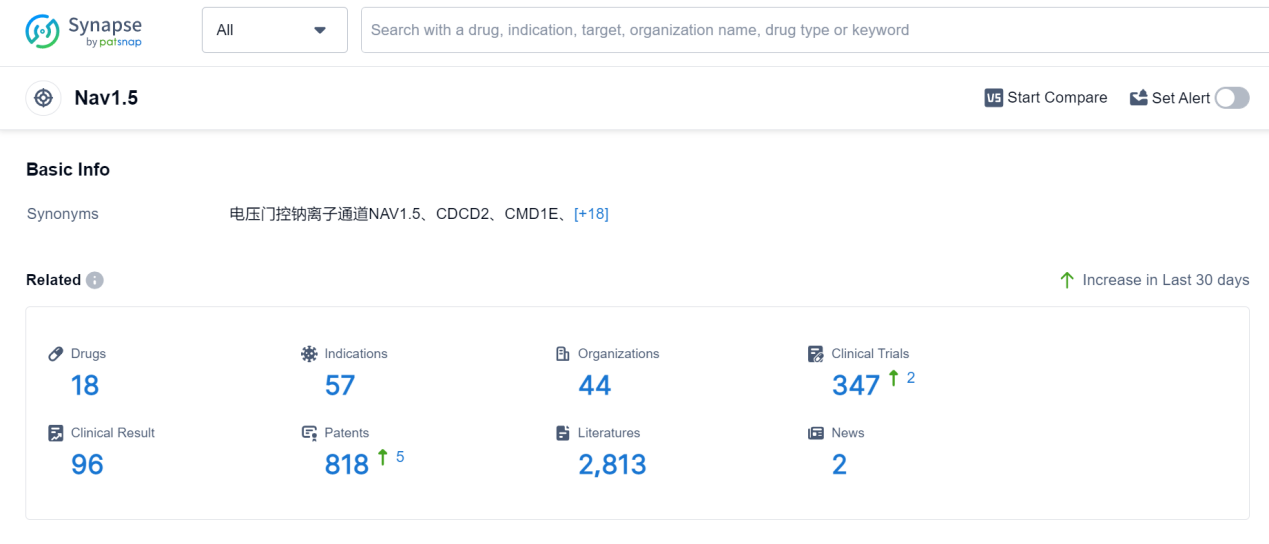
According to Patsnap Synapse, as of 7 Sep 2023, there are a total of 18 Nav1.5 drugs worldwide, from 44 organizations, covering 57 indications, and conducting 347 clinical trials.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
In conclusion, Ranolazine is a small molecule drug developed by Gilead Sciences, Inc. It has been approved for use in multiple therapeutic areas and has shown efficacy in treating various conditions. Its approval in the United States in 2006 marks its highest phase of development, indicating its successful completion of clinical trials. Ranolazine's targeting of Nav1.5 and its broad range of approved indications make it a promising drug in the field of biomedicine.