AbbVie Seeks FDA and EMA Approval for Upadacitinib (RINVOQ®) to Treat Giant Cell Arteritis
AbbVie revealed that it has filed applications with the U.S. Food and Drug Administration and the European Medicines Agency to approve a new use for upadacitinib (RINVOQ; 15 mg, once daily) aimed at the treatment of adult patients afflicted with giant cell arteritis.
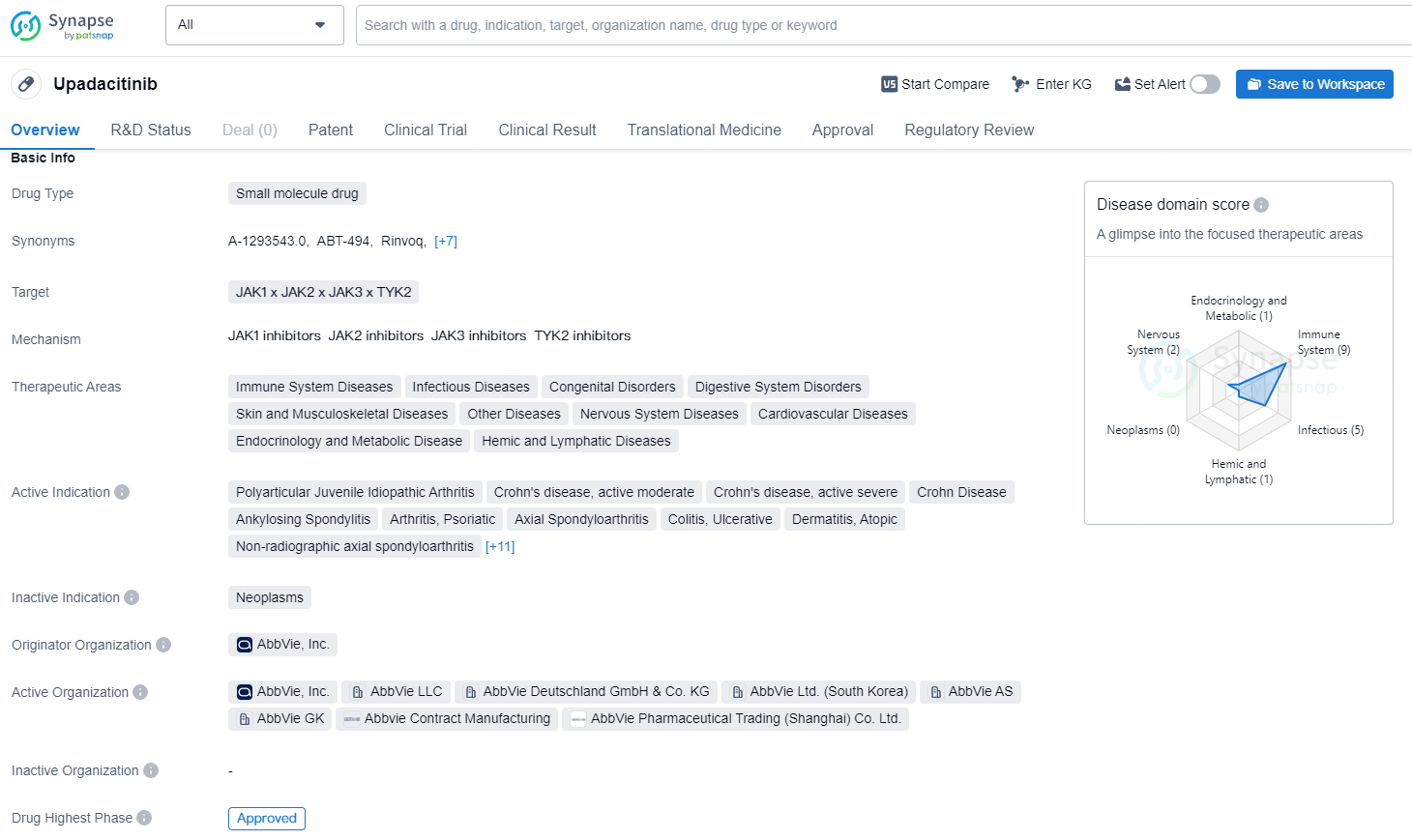
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
"At present, there are limited approved therapies for individuals with GCA. The majority of patients rely on glucocorticoids, yet many struggle to cease their use without experiencing a resurgence of GCA symptoms," said Roopal Thakkar, M.D., executive vice president of research and development and chief scientific officer at AbbVie. "We understand the significance of sustaining remission while minimizing glucocorticoid usage in GCA."
The submissions to both the FDA and EMA are backed by earlier results from the SELECT-GCA Phase 3 trial, which investigates the safety and effectiveness of upadacitinib in GCA patients.
SELECT-GCA is a Phase 3, multicenter, randomized, double-blind, placebo-controlled trial designed to assess the safety and efficacy of upadacitinib in 428 GCA patients. The trial includes two phases. The initial phase compared the efficacy of upadacitinib combined with a 26-week corticosteroid taper to a placebo combined with a 52-week corticosteroid taper.
Moreover, the initial phase also evaluated the safety and tolerability of upadacitinib in these patients. The ongoing second phase is assessing the safety and efficacy of continuing versus discontinuing upadacitinib in maintaining remission among participants who achieved sustained remission during the first phase.
Developed by AbbVie researchers, RINVOQ is a JAK inhibitor being explored for multiple immune-mediated inflammatory conditions. Enzymatic and cellular assays showed RINVOQ has higher inhibitory potency for JAK-1 vs JAK-2, JAK-3, and TYK-2.
The clinical relevance of inhibiting specific JAK enzymes for therapeutic efficacy and safety remains unknown. Upadacitinib is under investigation in Phase 3 trials for conditions including alopecia areata, giant cell arteritis, hidradenitis suppurativa, Takayasu arteritis, systemic lupus erythematosus, and vitiligo. Upadacitinib is not yet approved for use in giant cell arteritis, and its safety and efficacy for this condition have not been confirmed by regulatory bodies.
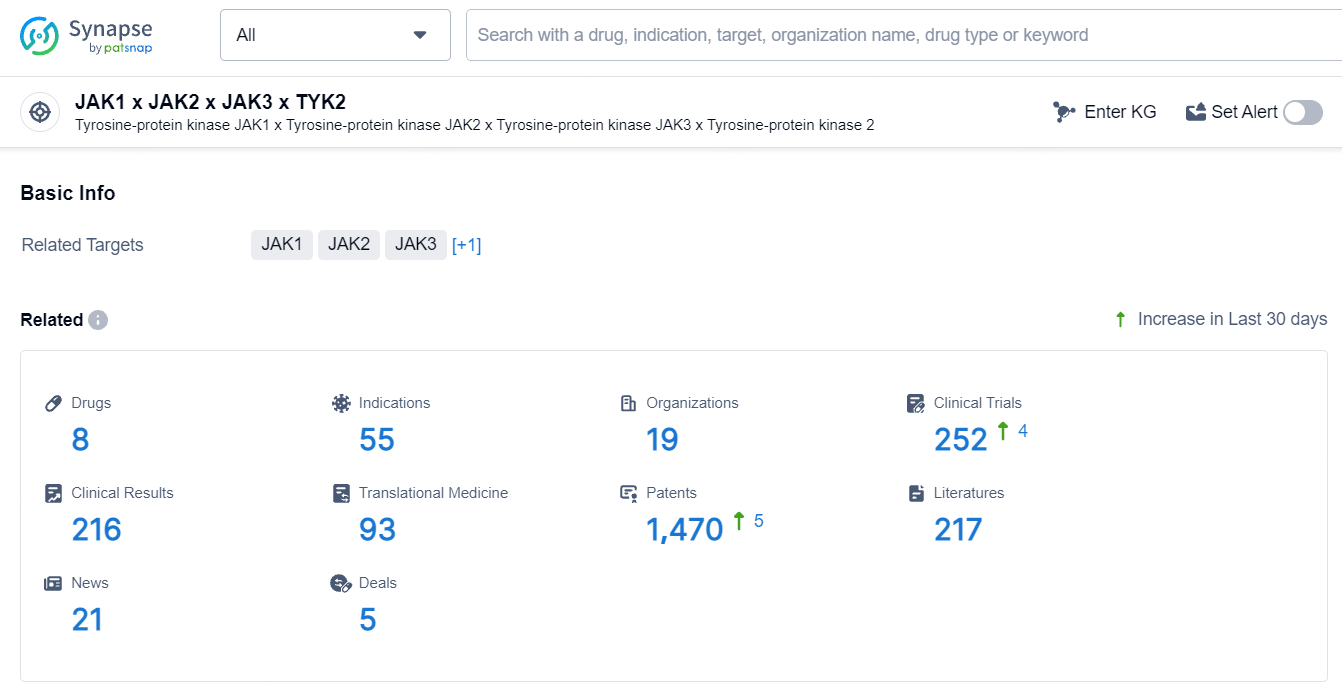
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of July 17, 2024, there are 8 investigational drugs for the JAK-1 vs JAK-2, JAK-3, and TYK-2 target, including 55 indications, 19 R&D institutions involved, with related clinical trials reaching 252, and as many as 1470 patents.
Upadacitinib shows promising potential in treating a wide range of diseases affecting different body systems, making it a valuable addition to the pharmaceutical market. Upadacitinib received its first approval in the United States in August 2019 and has since been granted Priority Review, Orphan Drug, Breakthrough Therapy, and Special Review Project designations. It is currently in the highest phase of development globally and in China.
How to obtain the latest research advancements in the field of biopharmaceuticals?
In the Synapse database, you can keep abreast of the latest research and development advances in drugs, targets, indications, organizations, etc., anywhere and anytime, on a daily or weekly basis. Click on the image below to embark on a brand new journey of drug discovery!