ASLAN Pharmaceuticals Provides TREK-DX Update, Highlights Eblasakimab's Success in Dupilumab-Treated Atopic Dermatitis Cases
ASLAN Pharmaceuticals Ltd., an enterprise specializing in immunological research at the clinical development phase, has revealed the initiation of patient recruitment within the United States, according to a revised protocol for its active TREK-DX study. This clinical trial is researching the effects of eblasakimab on individuals with moderate-to-severe atopic dermatitis who have previously been treated with dupilumab. The company is dedicated to discovering novel therapies with the goal of significantly improving patient health outcomes.
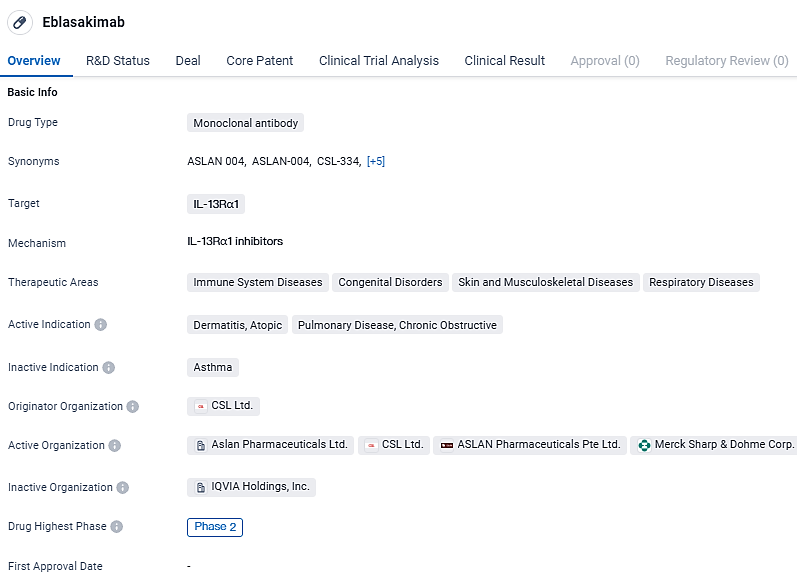
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
The inaugural TREK-DX study represents a pioneering effort as a randomized, double-masked study that uses a placebo control, focusing on a group of AD patients with prior dupilumab treatments. This segment is projected to approach a valuation of $10 billion within the next six years.
Insights gleaned from the TREK-AD investigation point to an evolving demographic of AD sufferers in the US, necessitating more selective entry conditions for TREK-DX. The study now requires participants to have a minimum Eczema Area and Severity Index (EASI) score of 18 for enrollment, an increase from the initial baseline of 16. Additionally, verification of these EASI scores by a third-party reviewer has been instituted.
TREK-DX aims to recruit around 75 participants who have stopped using dupilumab due to various reasons such as unsatisfactory AD management, limited accessibility to the medication, or experiencing negative side effects. These patients will undergo a 16-week regimen, receiving either a 400mg dose of eblasakimab or a placebo on a weekly basis. From the 22 individuals enrolled with the first set of criteria and following a 2:1 active-to-placebo randomization scheme, 17 successfully completed the prescribed 16-week treatment, while 5 discontinued earlier.
Eblasakimab specifically targets the IL-13 receptor (IL-13R) component of the Type 2 receptor, effectively inhibiting the signaling that involves both interleukin 4 and interleukin 13. The blockade of IL-13R has shown a notable capability in diminishing the levels of cytokines tied to Type 2 inflammation more effectively compared to interfering with IL-4R, and it also reduced the presence of Type 1 pro-inflammatory cytokines.
Furthermore, comparative data from direct studies juxtaposing eblasakimab with dupilumab, using skin biopsy samples from AD patients, substantiate the distinct impact of IL-13R targeting relative to IL-4R5. Eblasakimab was observed to more efficiently suppress the production of Type 2 pro-inflammatory cytokines in the skin compared to dupilumab, indicating that eblasakimab might offer a viable treatment alternative for AD patients who do not find dupilumab sufficiently efficacious.
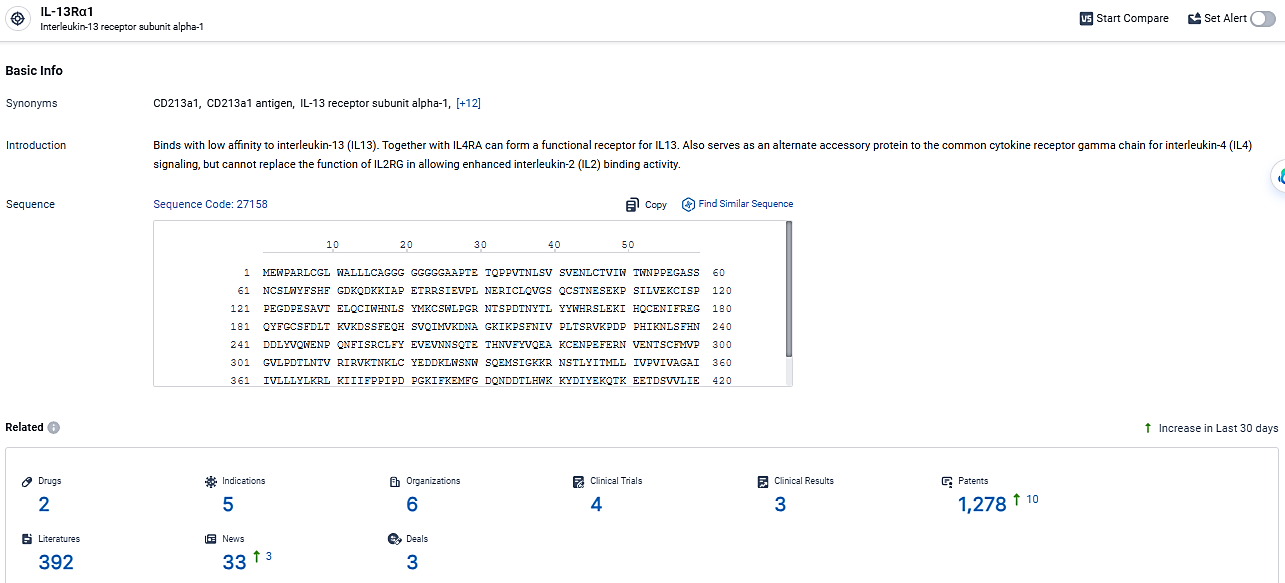
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of March 12, 2024, there are 2 investigational drugs for the IL-13Rα1 target, including 5 indications, 6 R&D institutions involved, with related clinical trials reaching 4, and as many as 1278 patents.
Eblasakimab targets IL-13Rα1 and has shown promise in treating immune system diseases, congenital disorders, skin and musculoskeletal diseases, and respiratory diseases. Currently in Phase 2, further clinical trials will be conducted to assess its safety and efficacy.