Absci Initiates Preclinical Trials for AI-Enhanced Anti-TL1A Candidate ABS-101
Absci Corporation, a pioneer in AI-driven drug discovery focusing on data-centric approaches, has revealed the commencement of studies aimed at supporting an Investigational New Drug (IND) application for ABS-101. This advanced candidate is an antibody targeting TL1A, meticulously crafted through the company's proprietary generative AI platform. ABS-101 is touted to emerge as a leading therapy within its class.
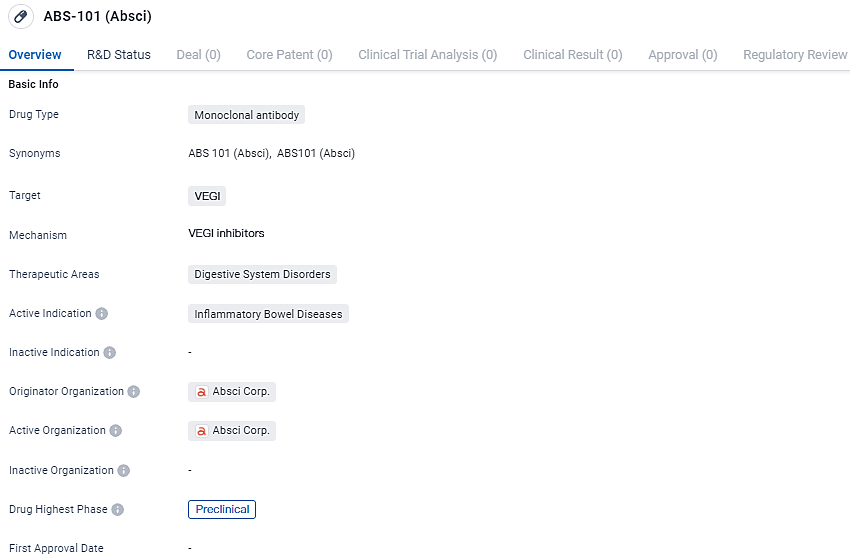
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
Using a defined target architecture, Absci employs its AI model to pinpoint an epitope of interest. This precision allows the company to craft antibodies that are epitope-specific, opening pathways to explore new biological phenomena. Absci then applies its advanced AI-driven optimization algorithms to enhance these antibody candidates, ensuring they possess ideal traits for successful clinical development.
At the onset of 2023, Absci revealed preliminary data from preclinical studies on ABS-101, indicating that their top three lead candidates exhibit characteristics suggesting they could surpass existing therapies, showcasing strong efficacy, high affinity, superior developability, and increased stability in the bloodstream.
The company tailored ABS-101 to an epitope, aided by innovative AI technology, aiming for heightened efficacy and reduced potential for immune response rejection. The envisioned profile for the product includes high bioavailability, which may lead to a more favorable administration routine for patients, requiring less frequent doses. After conducting additional pharmacokinetic (PK) research in February, Absci selected a prime candidate to proceed to IND (Investigational New Drug) preparatory research phases.
Sean McClain, the founder and chief executive of Absci, commented, "Embarking on the IND-preparatory studies for ABS-101 is a significant achievement for us. The advancement of this program illustrates our platform's capacity to rapidly generate a unique antibody therapeutic candidate - significantly faster than traditional industry timelines. We anticipate filing our IND application roughly two years post the project initiation."
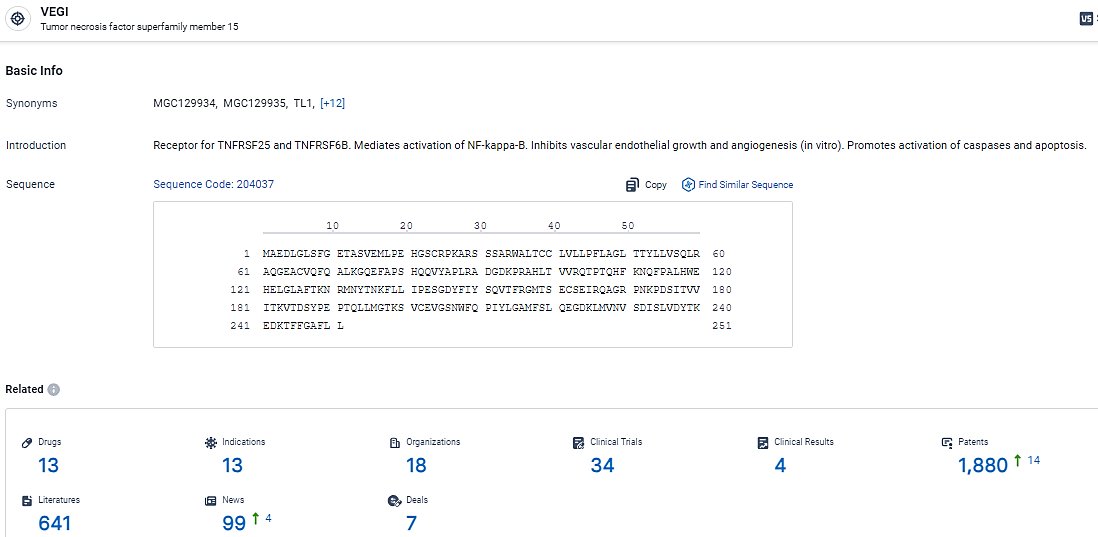
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of February 28, 2024, there are 13 investigational drugs for the VEGI target, including 13 indications,18 R&D institutions involved, with related clinical trials reaching 34, and as many as 1880 patents.
ABS-101 is a monoclonal antibody drug that targets VEGI and is being developed for the treatment of inflammatory bowel diseases in the therapeutic area of digestive system disorders. It is currently in the preclinical phase of development, and further research is needed to evaluate its potential as a treatment option for patients.