FDA Fast-Tracks Linvoseltamab Review for Relapsed/Refractory Multiple Myeloma
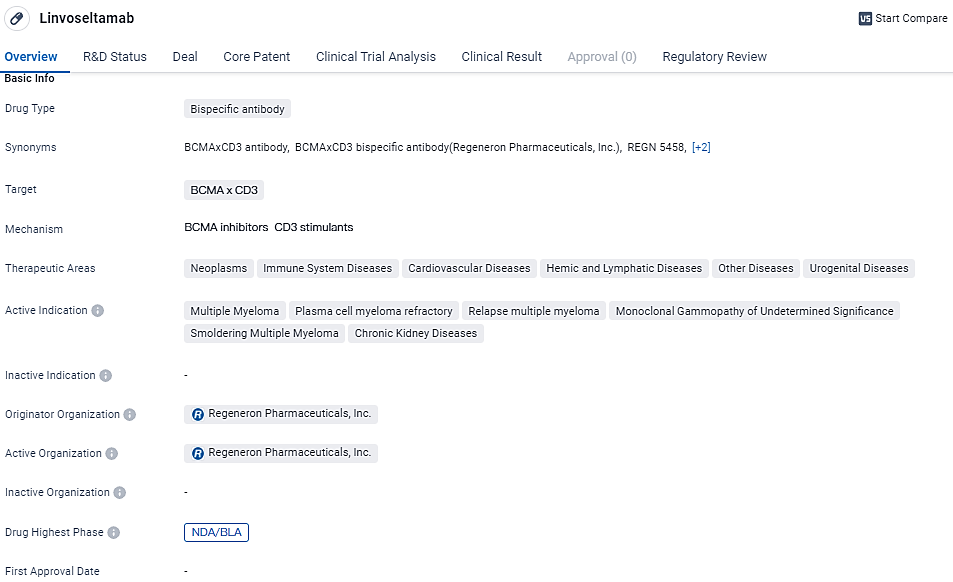
Regeneron Pharmaceuticals, Inc. has disclosed that the Biologics License Application for their drug candidate, linvoseltamab, intended for the treatment of adult individuals suffering from relapsed or refractory multiple myeloma following a minimum of three previous treatments, has been granted Priority Review status by the U.S. Food and Drug Administration.
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
The anticipated decision date by the FDA for Linvoseltamab is set for the 22nd of August in the year 2024. As a treatment under research, Linvoseltamab is a novel bispecific antibody that aims to connect the B-cell maturation antigen on myeloma cells with the CD3 receptors on T cells, thereby promoting the activation of T cells and destruction of cancer cells.
Data underpinning the Biologics License Application (BLA) comes from the critical Phase 1/2 study of Linvoseltamab in relapsed/refractory multiple myeloma (R/R MM), with the latest results disclosed in December of 2023. Additionally, the European Medicines Agency has initiated a review of the Marketing Authorization Application for Linvoseltamab targeting the same medical condition this month.
Multiple myeloma (MM) holds the position as the second-most prevalent hematologic malignancy. Statistics predict that 35,000 individuals may be diagnosed with MM within the United States annually. The disease manifests through uncontrolled growth of malignant plasma cells, which displace healthy cells in the bone marrow and can spread to other bodily tissues, causing serious harm to organs. Despite advancements in treatment, a cure for MM remains elusive. While existing therapies can decelerate the disease's progression, many patients will eventually witness the advancement of the condition and will need further treatment options.
The current research and development program for Linvoseltamab encompasses a Phase 3 study meant to serve as a confirmatory trial for patients battling R/R MM. This study is actively recruiting participants. Moreover, further studies are either being planned or are already in progress for earlier treatment phases and disease stages, including a Phase 1/2 study for initial treatment scenarios, a Phase 2 study focusing on patients with high-risk smoldering MM, and a Phase 2 study addressing monoclonal gammopathy of undetermined significance.
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
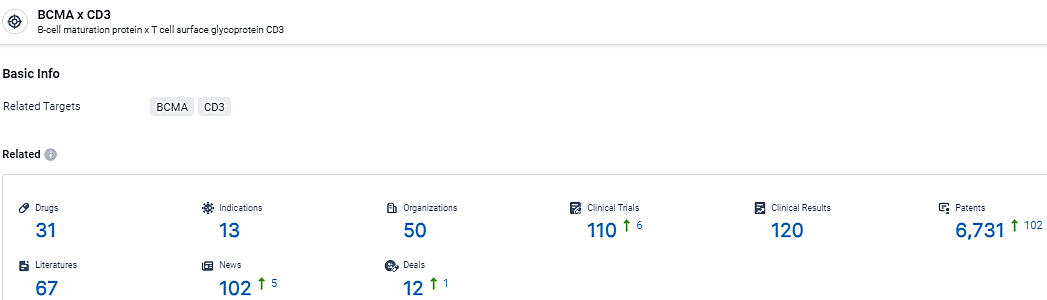
According to the data provided by the Synapse Database, As of February 28, 2024, there are 31 investigational drugs for the BCMA and CD3 target, including 13 indications,50 R&D institutions involved, with related clinical trials reaching 110, and as many as 6731 patents.
Linvoseltamab specifically targets BCMA and CD3 and has shown potential in treating multiple myeloma and other related conditions. With its current status at the NDA/BLA stage and the regulatory designations of priority review and fast track, Linvoseltamab is expected to undergo a thorough review process for potential approval and subsequent commercialization.