An analysis of Enzalutamide's R&D progress and its clinical results presented at the 2024 ASCO_GU Annual Meeting
On 25 Jan 2024, the outcome of enzalutamide combination treatment (tx) suspension in men with high-risk biochemically recurrent (BCR) prostate cancer was reported in the 2024 ASCO_GU.
Enzalutamide's R&D Progress
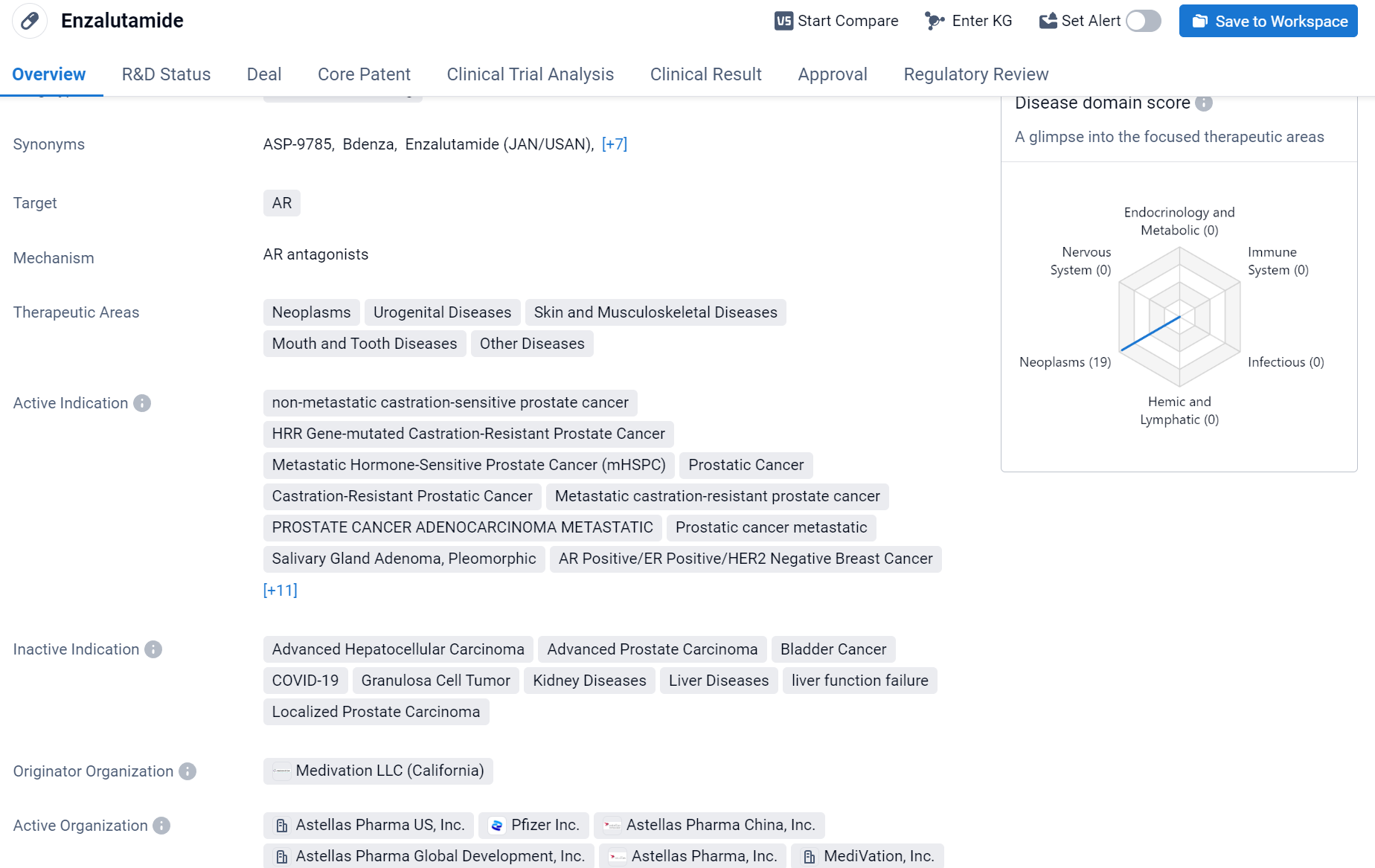
Enzalutamide is a small molecule drug that falls under the category of biomedicine. It primarily targets the androgen receptor (AR) and is used in the treatment of various diseases. The therapeutic areas in which Enzalutamide is utilized include neoplasms, urogenital diseases, skin and musculoskeletal diseases, mouth and tooth diseases, and other diseases.
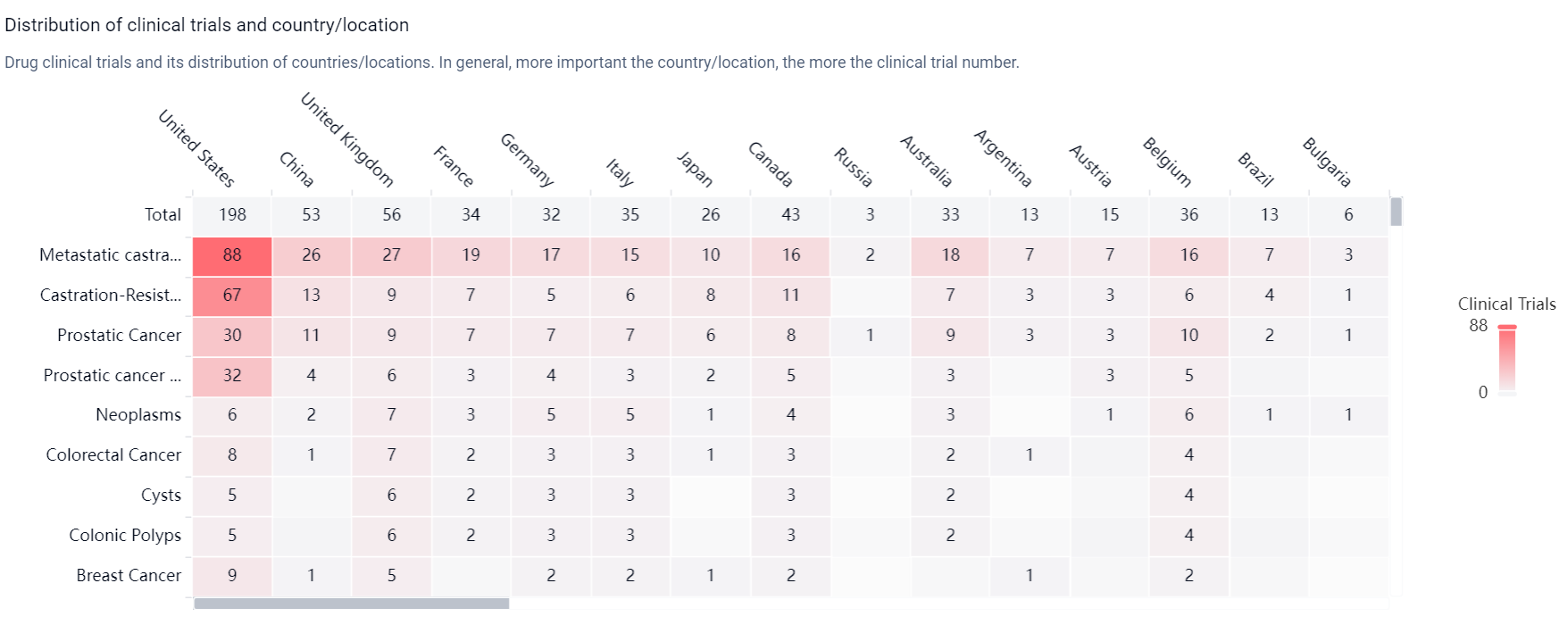
According to the Patsnap Synapse, Enzalutamide has received approval in multiple countries. And the clinical trial distributions for Enzalutamide are primarily in the United States, China and United Kingdom. The key indication is Metastatic castration-resistant prostate cancer.
Detailed Clinical Result of Enzalutamide
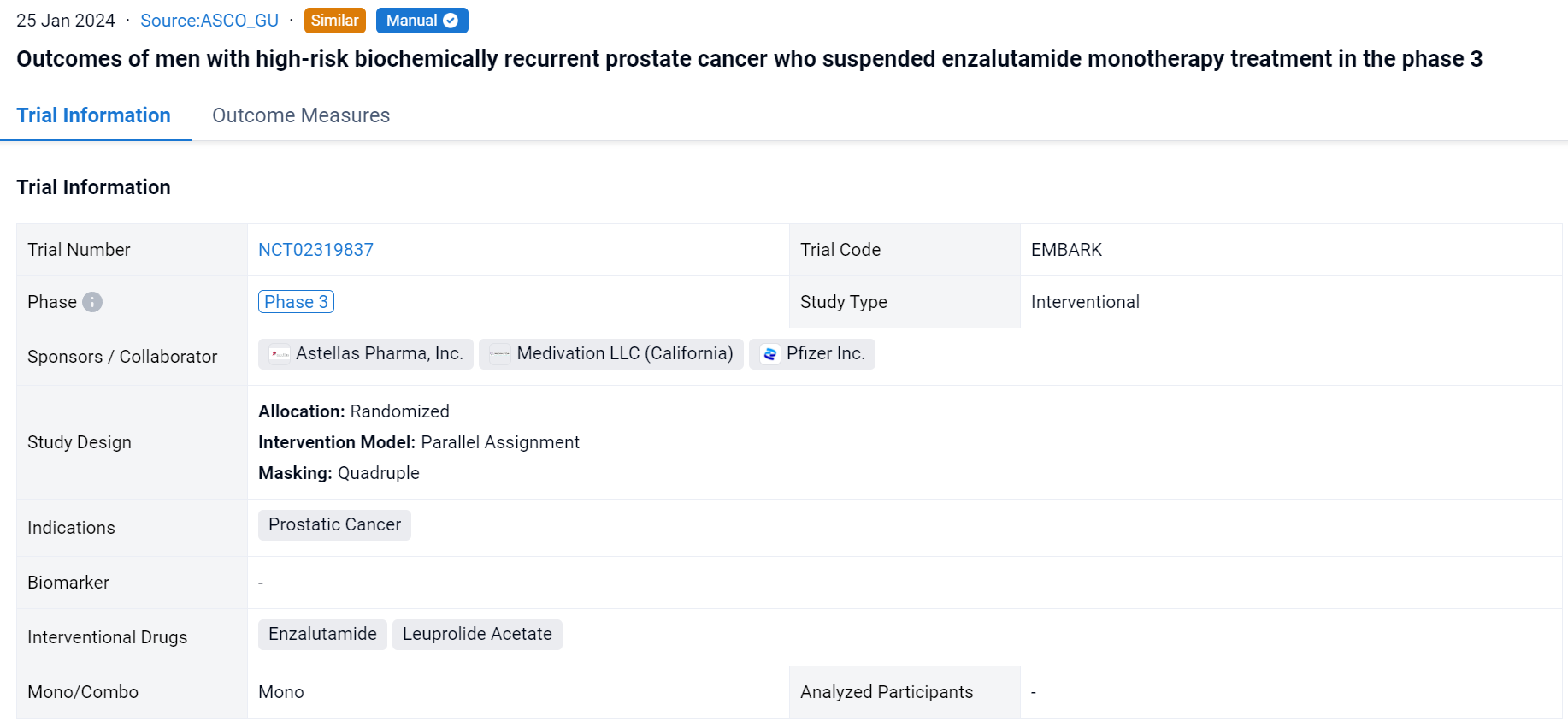
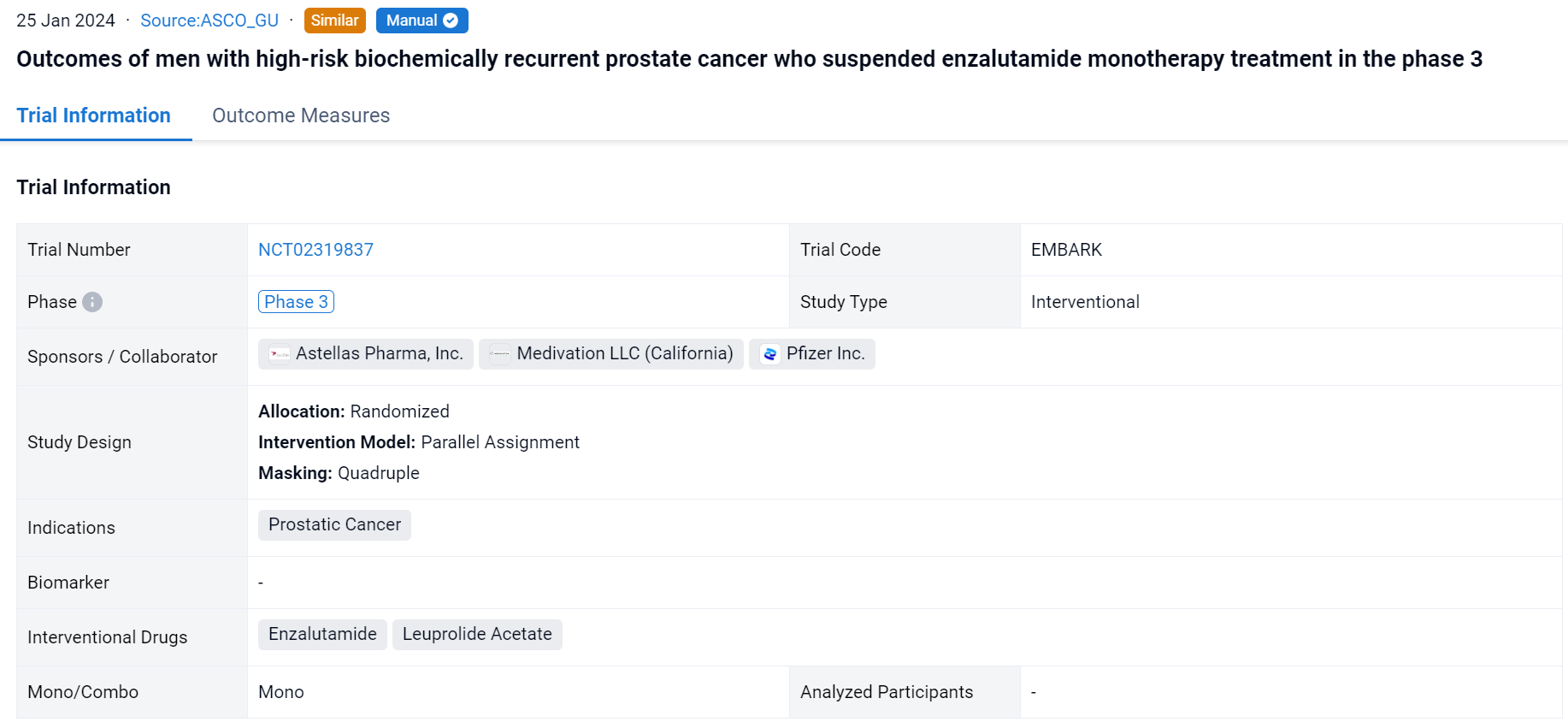
This randomized, parallel assignment, quadruple-blind clinical trial (NCT02319837) was aimed to evacuate the efficacy of enzalutamide + leuprolide acetate (enza combo) and enza monotherapy vs placebo + leuprolide (alone) on patients (pts) with high-risk BCR prostate cancer.
In this study, pts with high-risk BCR, defined as PSA doubling time ≤9 months, and PSA ≥2 ng/mL above nadir post-radiotherapy (RT) or ≥1 ng/mL after radical prostatectomy (RP) ± postoperative RT were enrolled. Pts were randomized 1:1 to enza 160 mg/day + leuprolide (22.5 mg every 12 weeks) or leuprolide alone. Tx was suspended at week 37 if serum PSA was <0.2 ng/mL at week 36 and restarted when PSA reached ≥2 ng/mL (primary RP) or ≥5 ng/mL (no primary RP). A secondary endpoint was the proportion of pts with undetectable PSA 2 years after suspension. MFS (per blinded independent central review) was analyzed descriptively between tx arms by suspension status.
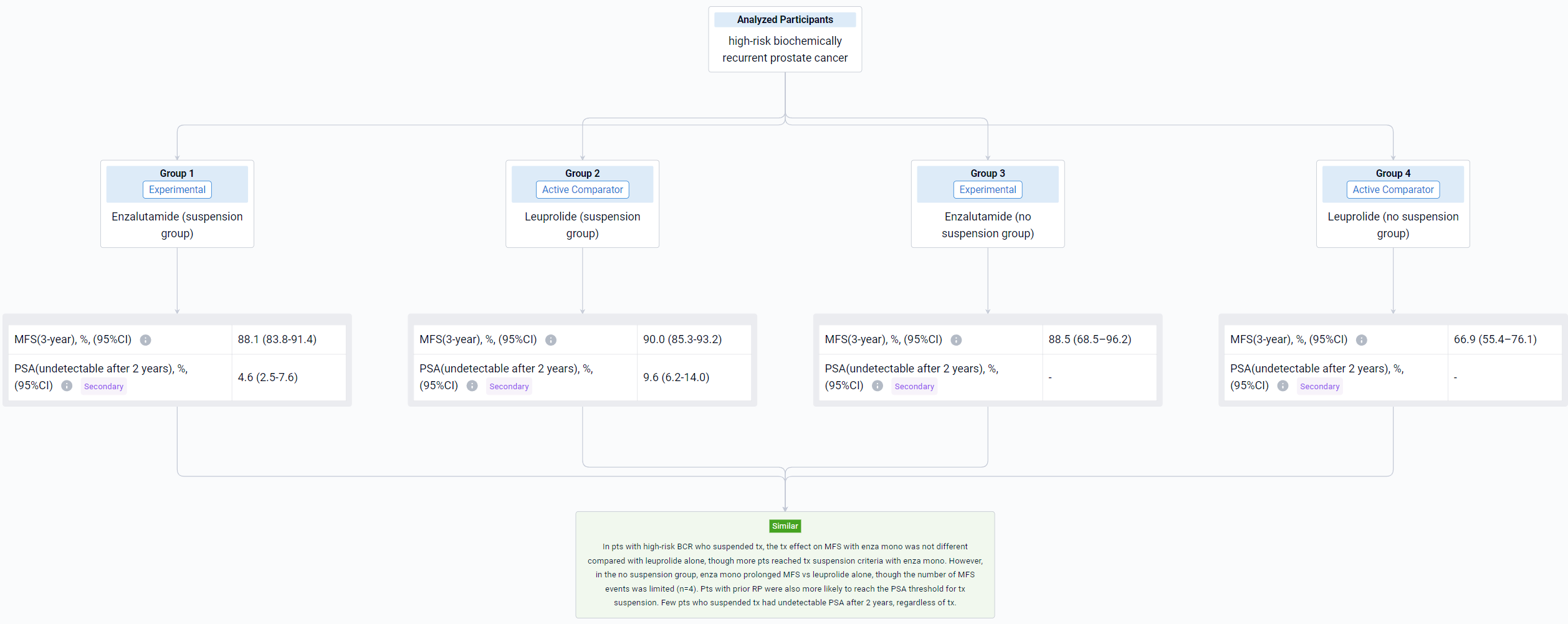
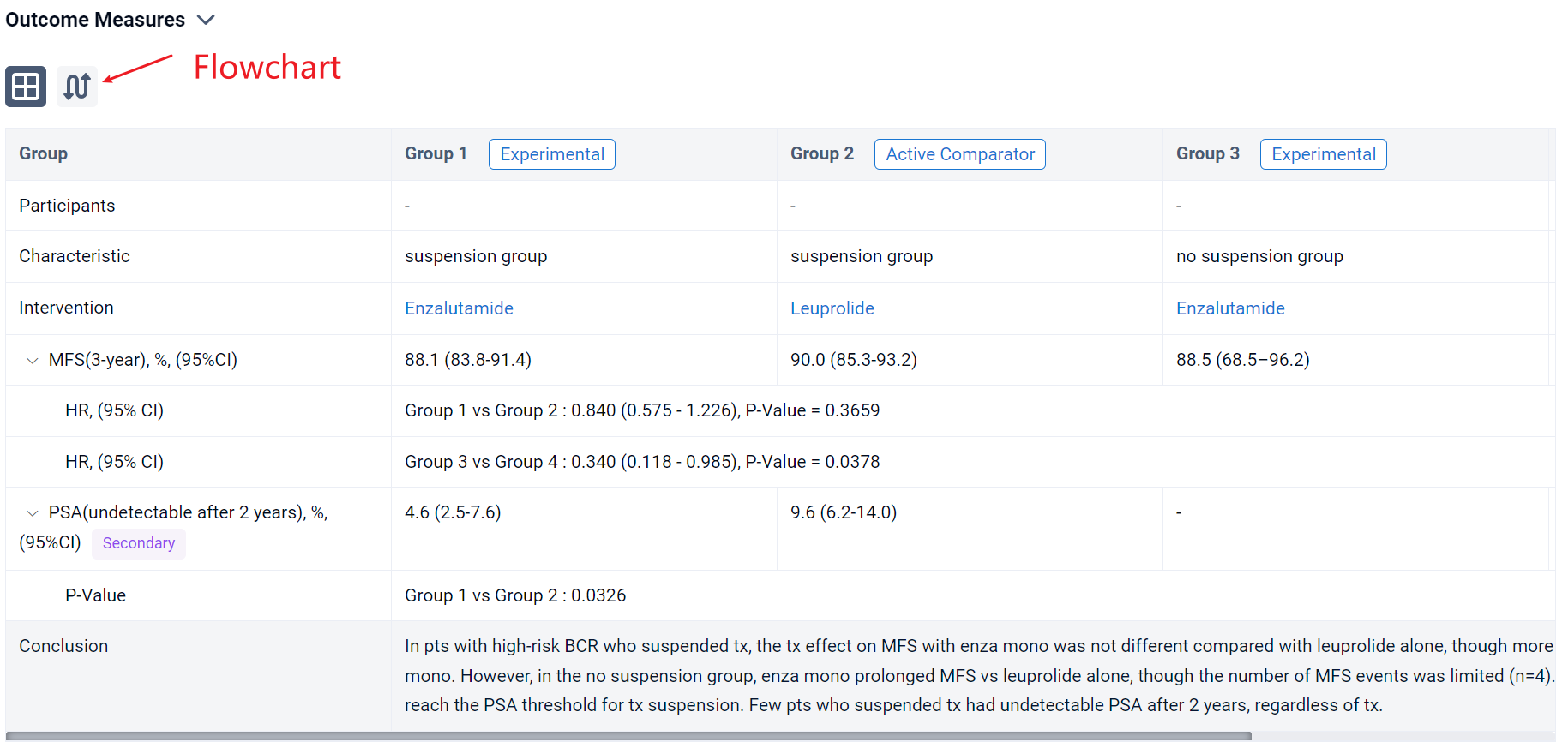
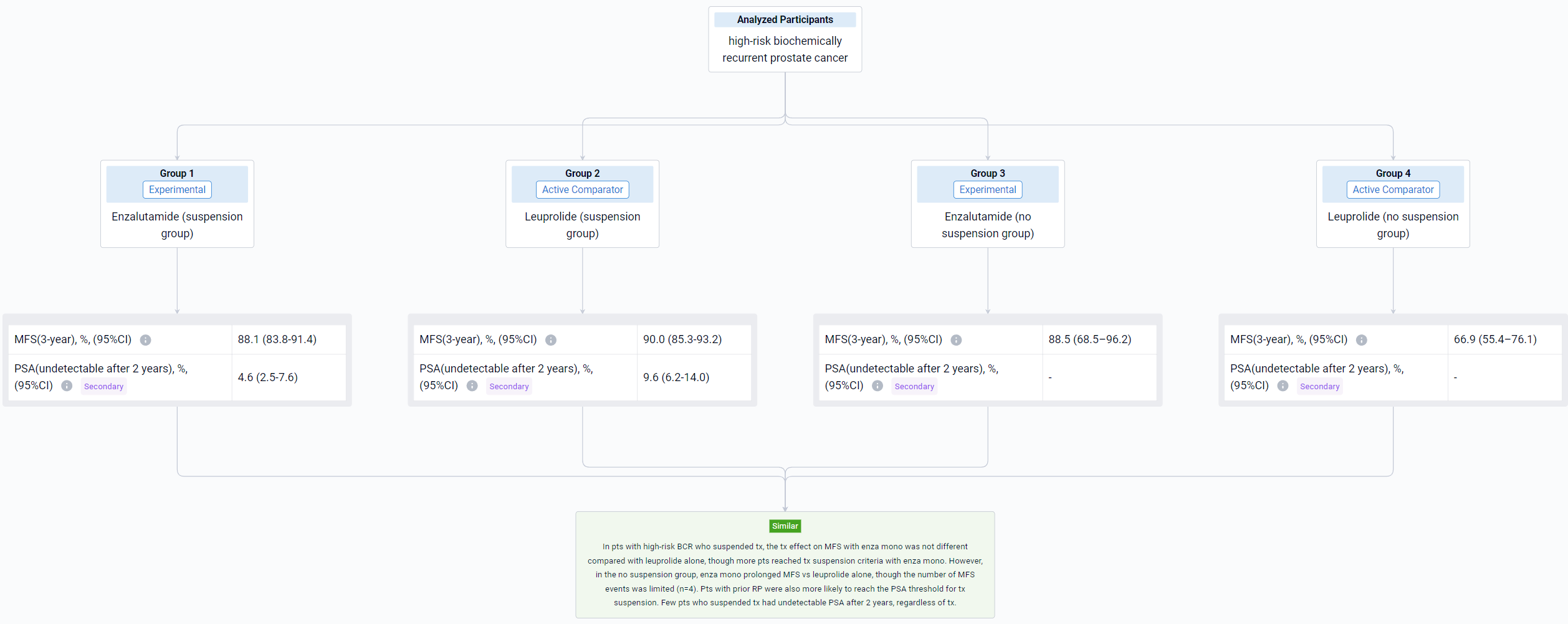
The result showed that the 3-year MFS rate (95% CI) in the suspension group was 94.4% (91.2–96.5%) with enza combo and 90.0% (85.3–93.2%) with leuprolide alone; in the no suspension group, 3-year MFS rates were 76.2% (33.2–93.5%) and 66.9% (55.4–76.1%), respectively. MFS in the suspension group was improved with enza combo vs leuprolide alone (HR 0.470, 95% CI 0.308–0.717; P=0.0003); no difference in MFS was observed in the no suspension group (HR 0.719, 95% CI 0.225–2.295; P=0.5763), although sample size was small for enza combo (n=9). Compared with no suspension, a higher proportion of pts in the suspension group received prior RP and a lower proportion received prior RT (Table). Two years after suspension, 16.8% (95% CI, 12.9–21.4%) of enza combo pts and 9.6% (95% CI, 6.2–14.0%) of leuprolide-alone pts had undetectable PSA (P=0.0089).
It can be concluded that MFS was improved with enza combo vs leuprolide alone for pts with BCR who suspended tx. No difference in MFS was observed in the no suspension group, but firm conclusions were precluded by the limited number of pts who did not suspend tx with enza combo. Pts who suspended tx had higher rates of prior RP vs those who did not. Pts who had received enza combo prior to tx suspension were more likely to have undetectable PSA 2 years thereafter compared with those who received leuprolide alone.
How to Easily View the Clinical Results Using Synapse Database?
If you want to know the other clinical results of popular conferences, please lick on the “Clinical Results” on the homepage of Patsnap Synapse, which provides multi-dimensional screening and filtering of drugs, indications, targets, companies, result evaluation, release date, popular conferences, etc. to help you quickly locate the data you need.
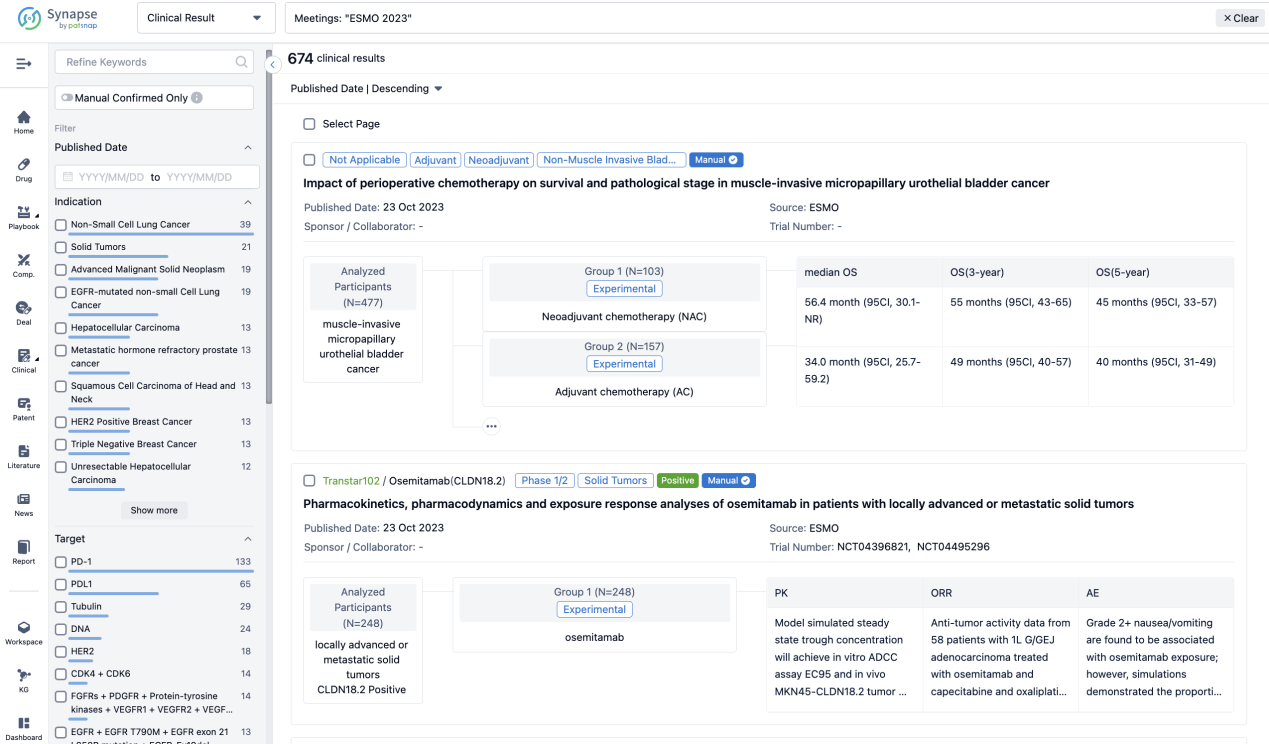
Select the clinical meeting you are interested in, such as ESMO. In the results, you can quickly locate the data you want to view by indication, phase and drug name.
A single result clearly shows important information such as registration number, phase, indication, Sponsor/Collaborator, biomarker, Trial number, dosing regimen and more.
If you would like to view more information about this result, you can go to the result detail page by clicking on the title.
Above the headings, we provide the original source of the outcome data. The basic information is supplemented with more information beyond the list, such as company, study. design, etc.
In the important Outcome Measures section, we provide both list and flowchart forms, which are convenient for you to overview the comparison group information and core indicator data.
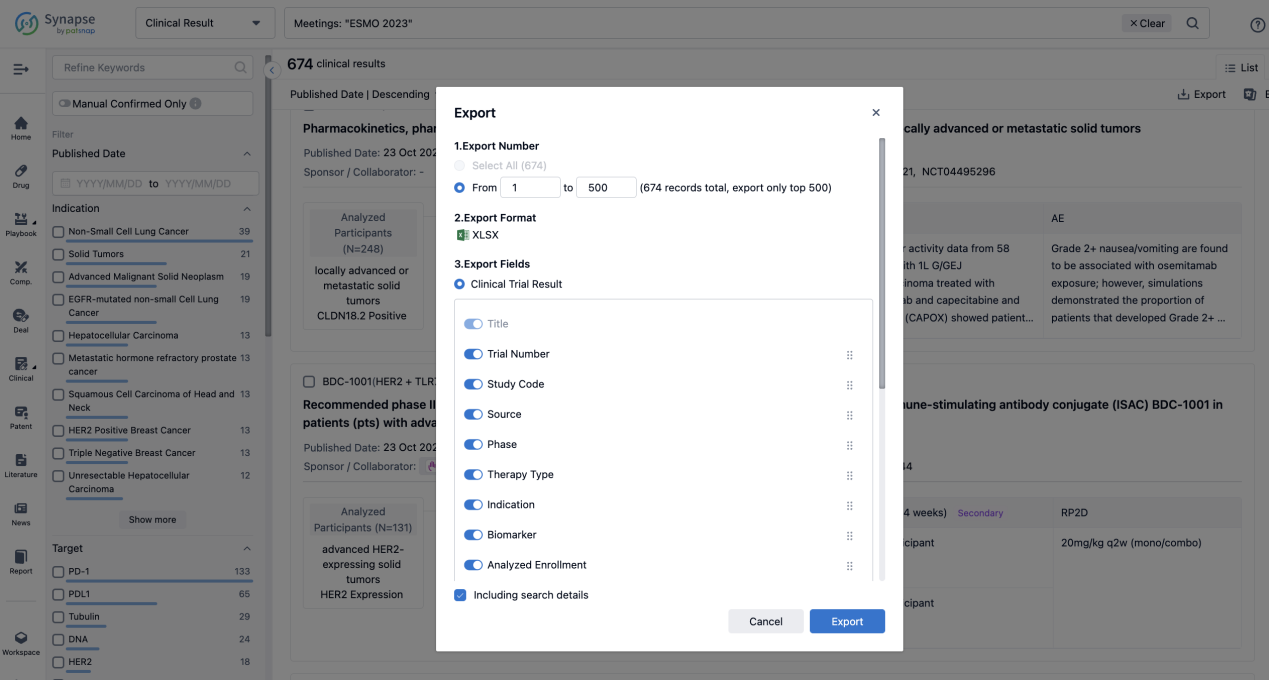
Finally, if you need to download these results, you can conveniently check the check boxes on the left side of the list, or directly click the "Export" button to download the data for personalized analysis and file sharing.
Click on the image below to embark on a brand new journey of drug discovery!