Actym Therapeutics Reveals IND Approval for Phase 1 Trial of ACTM-838 in Solid Tumor Patients
Actym Therapeutics revealed that the U.S. Food and Drug Administration has given the green light for its Investigational New Drug application, allowing the commencement of a Phase 1 clinical trial for its primary drug candidate, ACTM-838. This dose-escalation study, conducted as an open-label monotherapy, aims to assess the safety, tolerability, effective delivery, and initial anti-tumor effects of increasing doses of ACTM-838. This drug candidate is the pioneering therapy developed from the company's unique S.
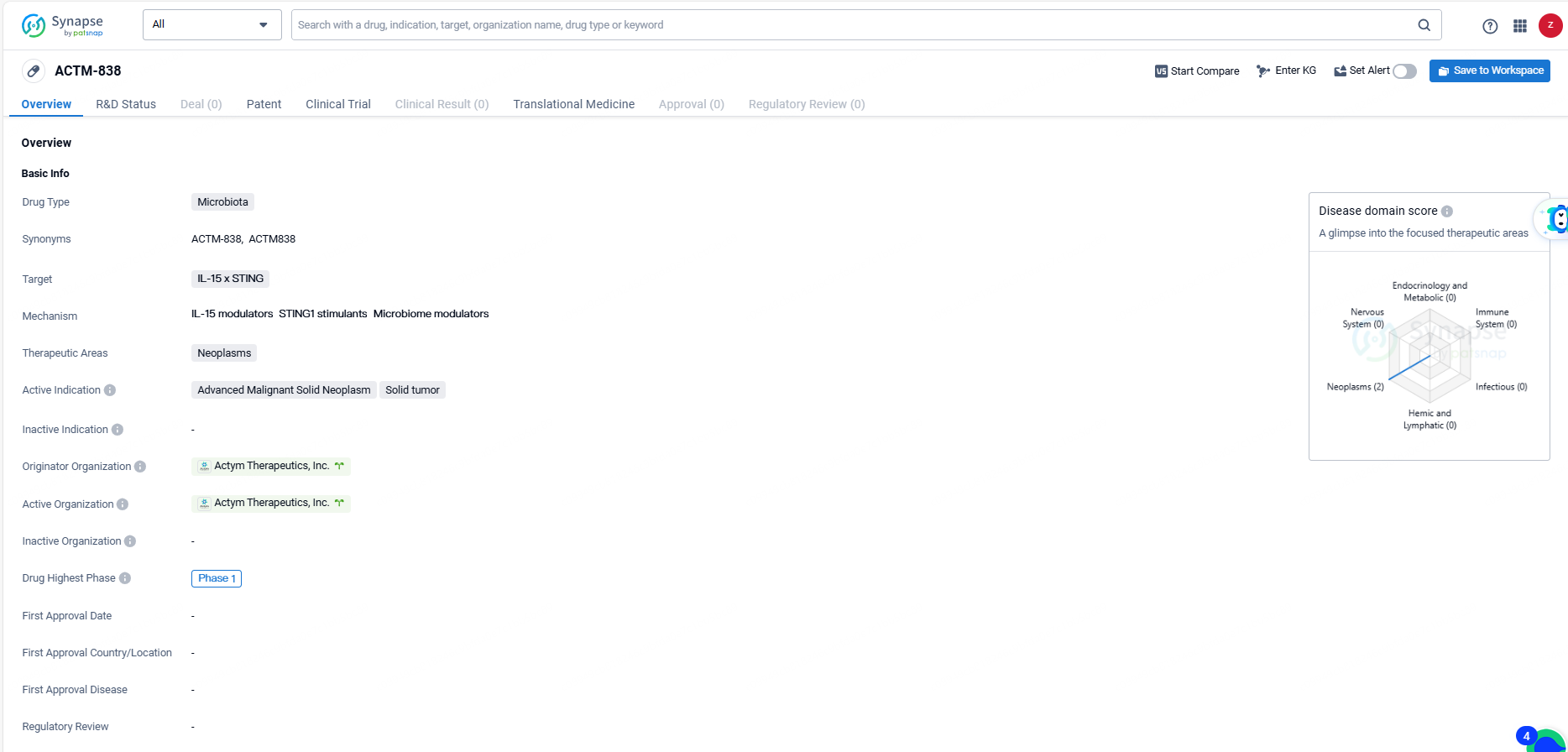
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
Typhimurium-Attenuated Cancer Therapy platform to enter clinical trials. ACTM-838 delivers immunomodulatory agents, including engineered IL-15plex (IL-15/ IL-15Ra) and STING, directly to phagocytic antigen-presenting cells located in the tumor microenvironment.
"The immunosuppressive nature of the tumor microenvironment (TME) poses a significant hurdle in the treatment of solid tumors, restricting the efficacy of numerous promising anticancer therapies," stated Jason J. Luke, MD, FACP, an internationally recognized physician-scientist specializing in the clinical development of immunotherapies for solid tumors. "Actym Therapeutics has introduced an advanced technology that precisely delivers and expresses active STING in tumor-resident myeloid cells through a systemic therapy."
Jason J. Luke further explained, "Actym’s strategy to modulate the TME holds unique promise to break through these immunological obstacles. Their leading candidate, ACTM-838, has demonstrated safety and efficacy in preclinical trials and is now poised to proceed to clinical evaluation."
"The IND approval for ACTM-838 represents a significant achievement for Actym and a promising step towards leveraging immunotherapy to more effectively treat patients with solid tumors," commented Tom Smart, CEO of Actym. "We are also eager to extend the potential uses of our STACT™ biological platform by developing new product candidates both independently and in collaboration with pharmaceutical companies."
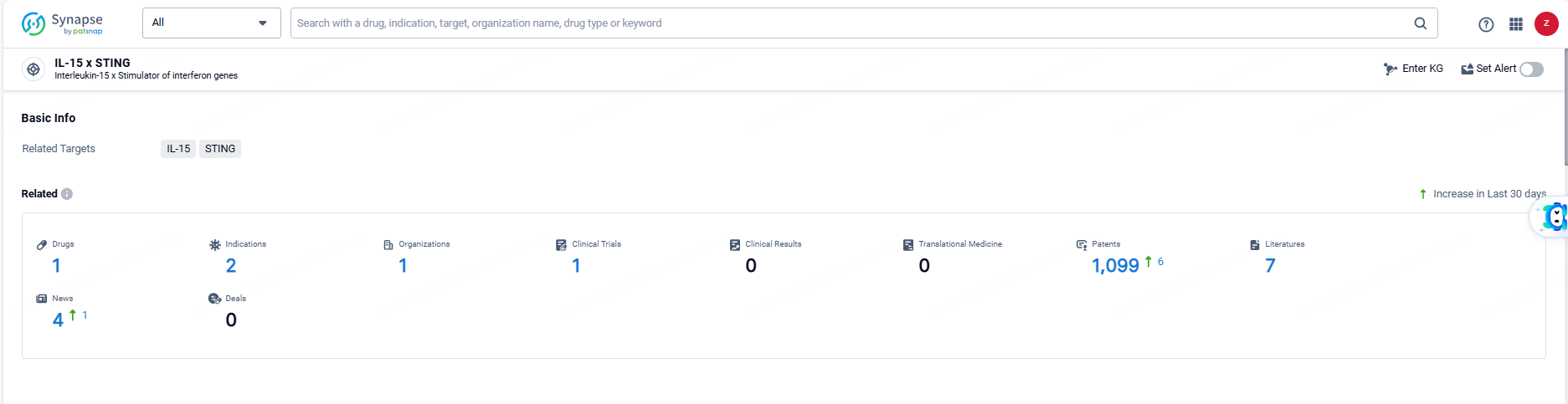
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of June 7, 2024, there are 1 investigational drugs for the IL-15 and STING target, including 2 indications, 1 R&D institutions involved, with related clinical trials reaching 1, and as many as 1099 patents.
ACTM-838 represents a microbiota drug targeting IL-15 x STING for the treatment of neoplasms, with a focus on advanced malignant solid neoplasms and solid tumors. Its current status at Phase 1 of clinical trials indicates early-stage development, and further research will be necessary to determine its potential as a therapeutic option for patients with these conditions.