Immunocore Unveils Phase 1 Brenetafusp (IMC-F106C) Results in Melanoma Patients at ASCO 2024
Immunocore Holdings plc, a biotechnology company at the commercial stage that is leading the development and distribution of groundbreaking immunomodulating treatments aimed at significantly enhancing patient outcomes for cancer, infectious diseases, and autoimmune conditions, has announced Phase 1 data involving its first off-the-shelf ImmTAC targeting PRAME, called brenetafusp (IMC-F106C). This data pertains to patients suffering from late-stage, post-checkpoint cutaneous melanoma. The findings reveal that brenetafusp is well tolerated both as a monotherapy and when combined with anti-PD1, and it shows sustained clinical benefits.
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
"Brenetafusp continues to show encouraging monotherapy clinical efficacy in patients with advanced cutaneous melanoma who have previously undergone checkpoint therapy treatments," stated Dr. Omid Hamid, Chief of Translational Research and Immunotherapy and Co-Director of Melanoma Therapeutics at Cedars-Sinai Cancer, The Angeles Clinic and Research Institute. "The observed disease control rate and progression-free survival (PFS) in melanoma patients treated with brenetafusp compare favorably with results from other immunotherapy treatments."
"The key indicator of brenetafusp's monotherapy effectiveness is disease control, which we have seen in 56% of melanoma patients who had prior checkpoint therapy," added David Berman, Head of Research and Development. "Our analysis of blood T cell health supports the expectation that brenetafusp's PFS could be even greater in first-line therapy. These findings, alongside the strong molecular response and the expected synergistic benefits when combined with an active anti-PD1 agent, bolster our confidence in PFS as a primary endpoint in our ongoing Phase 3 first-line trial."
Brenetafusp was generally well-tolerated, with manageable treatment-related adverse events consistent with its mechanism of action. The most commonly reported treatment-related adverse events (TRAEs) were Grade 1 or 2 cytokine release syndrome (CRS) and rash, occurring mainly after the first three doses. No Grade 3 or higher CRS TRAEs were reported.
IMC-F106C-101 is a first-in-human Phase 1/2 dose-escalation study involving patients with various solid tumors, including non-small cell lung cancer, small cell lung cancer, endometrial cancer, ovarian cancer, cutaneous melanoma, and breast cancer. The Phase 1 dose-escalation phase aimed to determine the maximum tolerated dose, assess the safety, preliminary anti-tumor efficacy, and pharmacokinetics of IMC-F106C (brenetafusp), a bispecific protein developed using Immunocore’s ImmTAC technology, and the Company's inaugural molecule targeting the PRAME antigen.
The Company has commenced patient enrollment for four expansion cohorts focusing on cutaneous melanoma, ovarian cancer, NSCLC, and endometrial carcinoma. The adaptive IMC-F106C-101 trial includes a Phase 2 expansion option, with approximately 100 patients per tumor type receiving treatment in the combined Phase 1 and 2 expansion cohorts. Phase 1 monotherapy investigations are ongoing in additional solid tumors, along with multiple combination therapies, including checkpoint inhibitors, chemotherapy, targeted therapies, and tebentafusp.
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
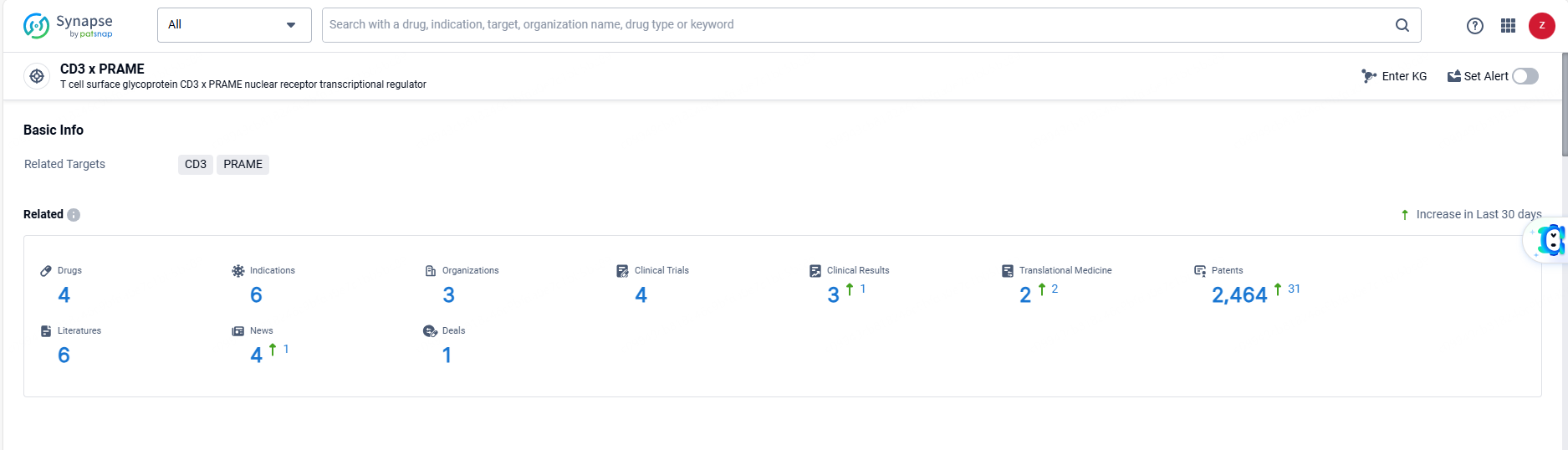
According to the data provided by the Synapse Database, As of June 7, 2024, there are 4 investigational drugs for the CD3 and PRAME targets, including 6 indications, 3 R&D institutions involved, with related clinical trials reaching 4, and as many as 2464 patents.
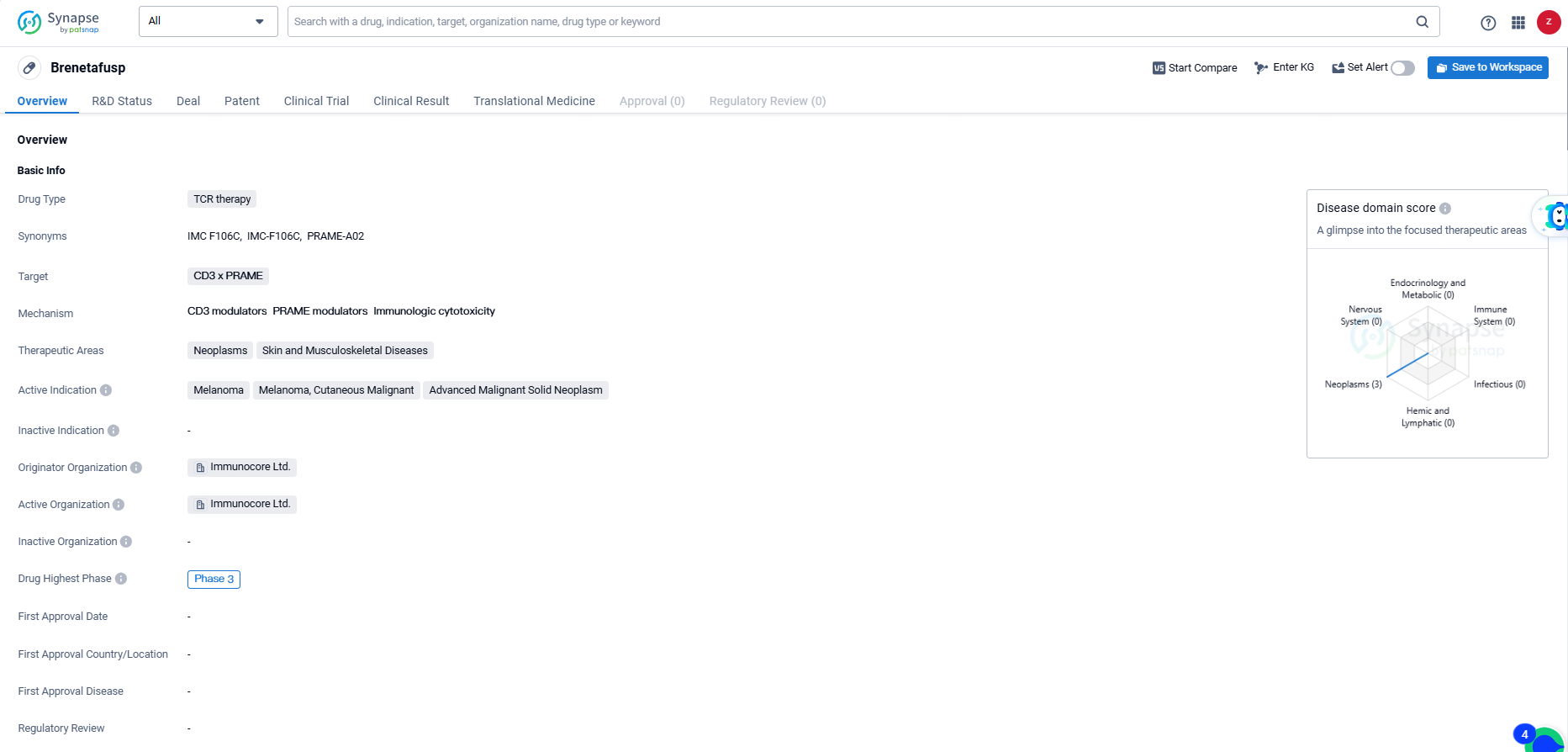
Brenetafusp is a TCR therapy drug developed by Immunocore Ltd. The drug targets CD3 x PRAME and is primarily focused on treating neoplasms, skin, and musculoskeletal diseases. The active indications for Brenetafusp include melanoma, cutaneous malignant melanoma, and advanced malignant solid neoplasm.