ACE2 - The Human Cell Receptor "Turned Traitor" by COVID-19 Virus
Angiotensin-converting enzyme 2 (ACE2) is a key enzyme in the Renin-Angiotensin System (RAS), acting as a regulating factor for the ACE-Angiotensin II (Ang II)-Ang receptor I (AT1) axis, maintaining the balance of the RAS system. ACE2 is classified as a type I transmembrane glycoprotein (with the C-end being inside the cell). The ACE2 gene is located on the X22 chromosome in humans, containing 18 exons. The complete protein comprises 805 amino acids and ACE2 primarily exists on the cytoplasmic membrane in the form of a membrane-bound protein. The extracellular domain of ACE2 includes a catalytic metalloprotease structural region (zinc-binding domain).
Traditionally, it was believed that ACE2 is mainly expressed in the heart, kidneys, and testes. However, later studies found that ACE2 is also expressed in the jejunum, duodenum, cecum, ileum, and other parts of the digestive system, as well as in the lungs, bone marrow, spleen, liver, retina, placenta, ovaries, brain tissue, adipose tissue, macrophages, and various other tissue cells.
Originally, ACE2 is a normal structure in the human body. However, after binding with the receptor-binding domain (RBD) of the S protein of the novel coronavirus, it has become an essential step for the virus to infect the human body. By binding to ACE2, the virus gains entry into human cells, particularly in the respiratory system. Understanding the role of ACE2 is essential for developing therapeutic interventions and vaccines to combat COVID-19 and other related diseases.
ACE2 Competitive Landscape
According to the data provided by Patsnap Synapse-Global Drug Intelligence Database: the following figure shows that as of 7 Sep 2023, there are a total of 16 ACE2 drugs worldwide, from 17 organizations, covering 7 indications, and conducting 6 clinical trials.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, organizations, clinical trials, clinical results, and drug patents related to this target.
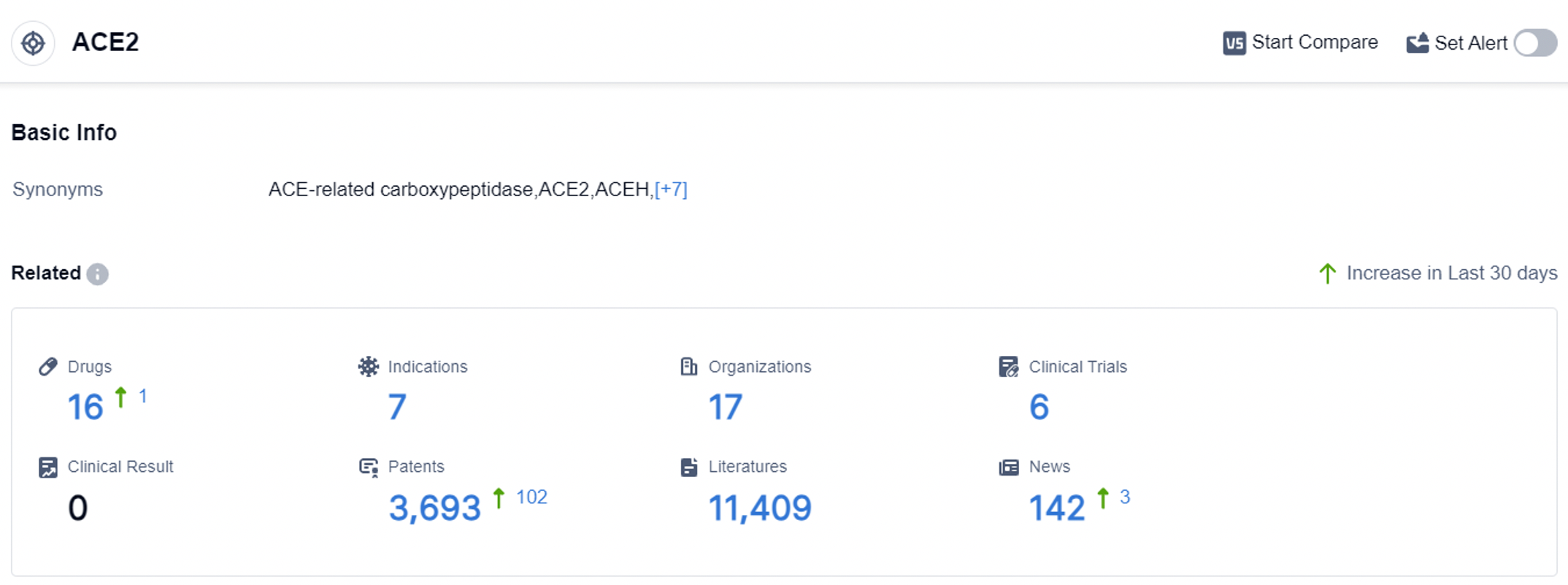
The analysis of the target ACE2 reveals a competitive landscape with multiple companies actively engaged in research and development. The indications being targeted include hypertension, acute lung injury, COVID-19, and coronavirus infections, reflecting the urgent need to address these conditions. The drug types being explored range from small molecules and proteins to mRNA-based drugs and various other innovative approaches.
China and the United States are leading the development efforts, with other countries also actively participating. The future development of target ACE2 holds promise for addressing critical medical needs and advancing therapeutic options in the pharmaceutical industry.
Key Drug: ACV-200-17
ACV-200-17 is a protein drug that targets ACE2, MSN, and the SARS-CoV-2 S protein. It is primarily developed for the treatment of COVID-19, focusing on infectious and respiratory diseases. The drug is currently in the clinical phase globally, indicating that it has progressed through earlier stages of development and is being tested in human subjects. However, in China, it is still in the preclinical phase, suggesting that it is undergoing laboratory testing and animal studies before advancing to human trials.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
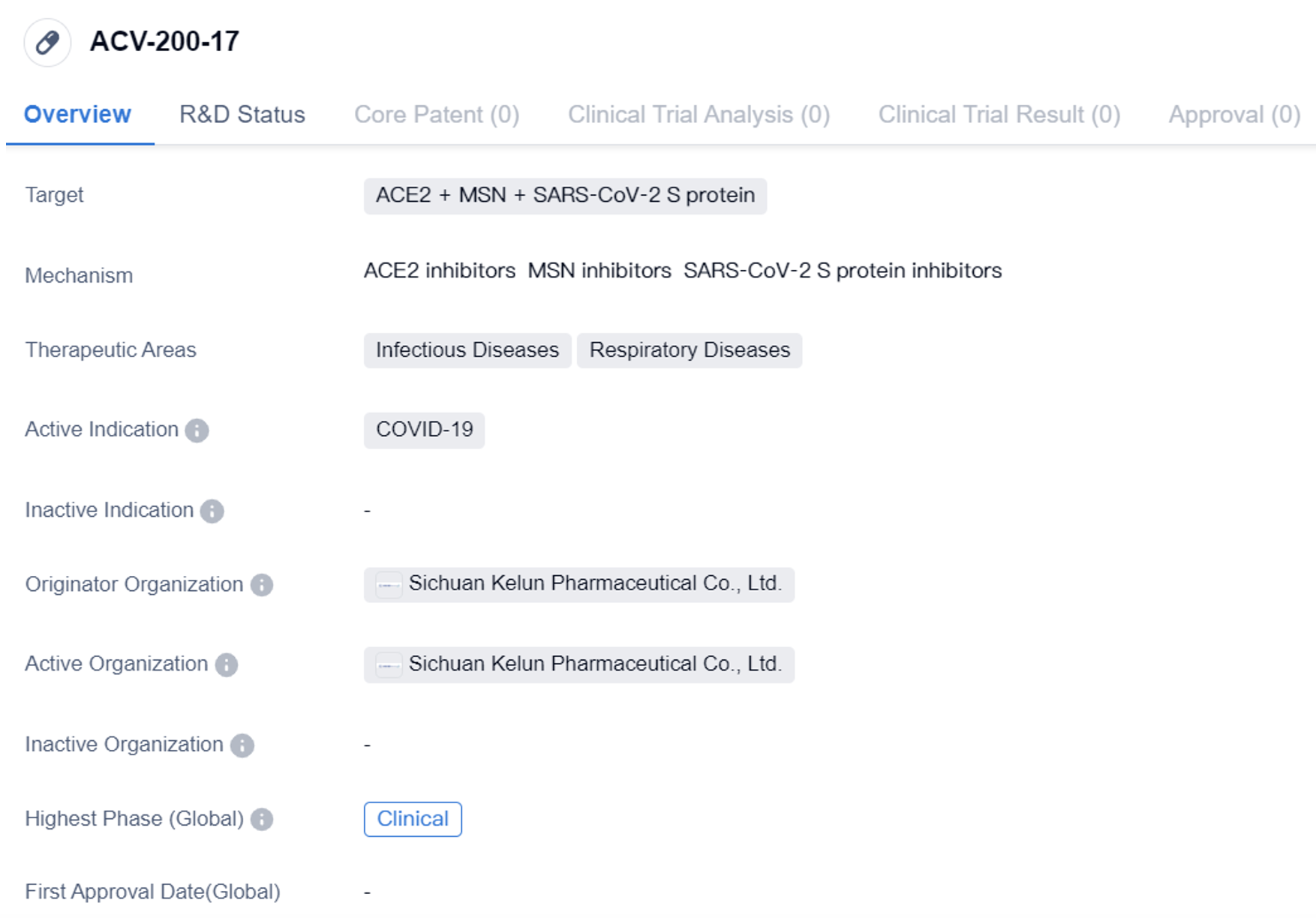
Protein drugs are a type of pharmaceutical product that utilizes proteins, such as antibodies or enzymes, to target specific molecules or pathways in the body. In the case of ACV-200-17, it targets ACE2, MSN, and the SARS-CoV-2 S protein. ACE2 is an enzyme involved in regulating blood pressure, and it has been identified as the receptor that allows the SARS-CoV-2 virus to enter human cells. MSN, on the other hand, refers to mesenchymal stem cells, which have shown potential in treating various diseases due to their regenerative properties.
In summary, ACV-200-17 is a protein drug developed by Sichuan Kelun Pharmaceutical Co., Ltd. It targets ACE2, MSN, and the SARS-CoV-2 S protein, with a focus on treating COVID-19 and related infectious and respiratory diseases. While it has reached the clinical phase globally, it is still in the preclinical phase in China. Further information regarding the drug's progress, efficacy, and safety is not provided in the given information.