Rilonacept Unveiled: A Detailed Overview of its Revolutionary R&D Breakthroughs
Rilonacept's R&D Progress
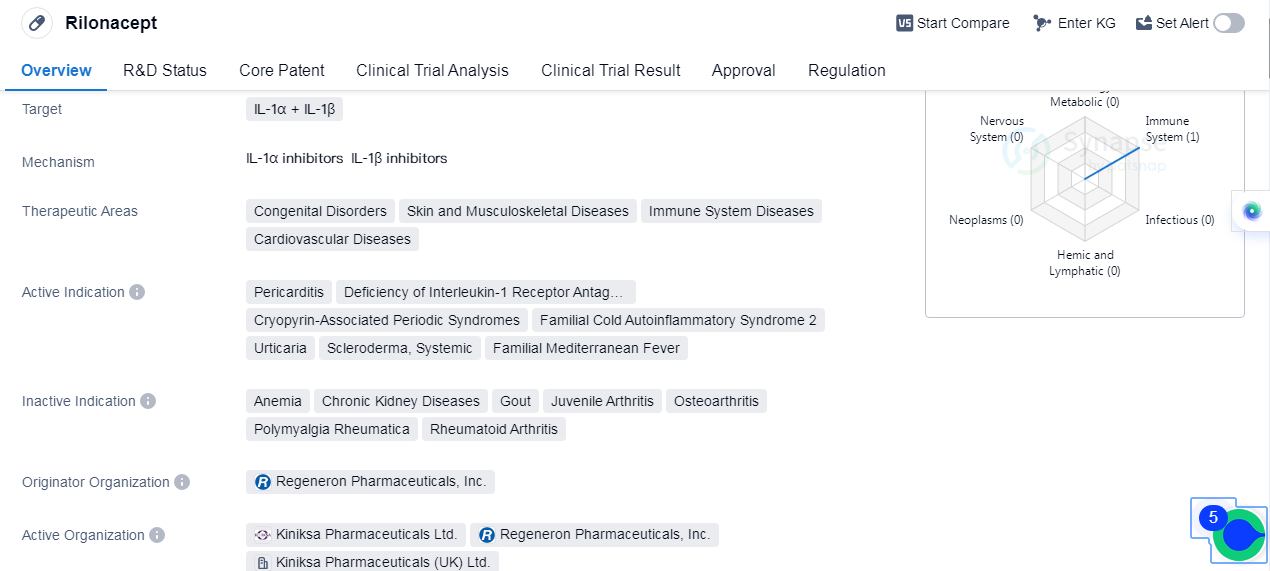
Rilonacept is a fusion protein drug that targets IL-1α and IL-1β. It is primarily used in the treatment of congenital disorders, skin and musculoskeletal diseases, immune system diseases, and cardiovascular diseases. The drug has been approved for the treatment of pericarditis, deficiency of interleukin-1 receptor antagonist, cryopyrin-associated periodic syndromes, familial cold autoinflammatory syndrome 2, urticaria, scleroderma, systemic diseases, and familial Mediterranean fever.
Rilonacept was developed by Regeneron Pharmaceuticals, Inc., an originator organization in the pharmaceutical industry. The drug has reached the highest phase of development, which is approved globally. However, in China, it is still in the discovery phase.
The first approval of Rilonacept took place in the United States in February 2008. It received a priority review, indicating its potential to address an unmet medical need. Additionally, it has been classified as an overseas new drug urgently needed in clinical settings, suggesting its importance in international markets. The drug has also been granted fast-track designation, indicating its potential to treat serious conditions and improve patient outcomes.
Furthermore, Rilonacept has been designated as an orphan drug, indicating its use in the treatment of rare diseases. It has also received breakthrough therapy designation, highlighting its potential to provide significant benefits over existing treatments.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Rilonacept: Inhibitor of IL-1α and IL-1β
Inhibitors of Interleukin-1 alpha (IL-1α) and Interleukin-1 beta(IL-1β)target and inhibit the activity of IL-1α and IL-1β, respectively.
IL-1 is a cytokine, which is a type of signaling molecule involved in the immune response and inflammation. IL-1α and IL-1β are two forms of IL-1 that play important roles in the regulation of immune and inflammatory processes in the body.
IL-1α and IL-1β are produced by various cells, including immune cells.They bind to specific receptors on target cells to initiate a cascade of immune and inflammatory responses. However, excessive or dysregulated IL-1α and IL-1β can contribute to the development and progression of various diseases, including autoimmune disorders, chronic inflammatory conditions, and certain types of cancer.
IL-1 inhibitors, such as IL-1α inhibitors and IL-1β inhibitors, work through blocking the activity of IL-1α or IL-1β, thereby reducing the immune and inflammatory responses mediated by these cytokines. By inhibiting IL-1α or IL-1β, these medications can help alleviate symptoms and slow down the progression of diseases associated with excessive IL-1 activity.
These inhibitors are used in the treatment of various conditions, including rheumatoid arthritis, systemic juvenile idiopathic arthritis, and certain autoinflammatory diseases. They are typically administered through injections or infusions and are prescribed under the guidance of a healthcare professional.
Overall, IL-1α and IL-1β inhibitors are important therapeutic agents that target specific cytokines involved in immune and inflammatory processes, providing relief for patients with certain diseases.
Drug Target R&D Trends for Rilonacept
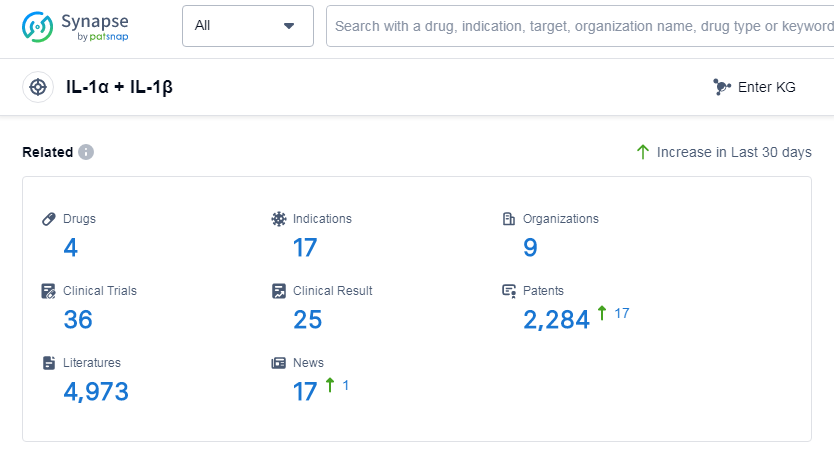
According to Patsnap Synapse, as of 1 Sep 2023, there are a total of 4 IL-1α and IL-1β drugs worldwide, from 9 organizations, covering 17 indications, and conducting 36 clinical trials.
In conclusion, the analysis of the target IL-1α and IL-1β reveals that Regeneron Pharmaceuticals, Inc. and Kiniksa Pharmaceuticals Ltd. are the companies growing fastest in this field. The drugs under this target have been approved for indications such as cryopyrin-associated periodic syndromes, pericarditis, and familial cold autoinflammatory syndrome 2. Fusion proteins are the most common drug type, indicating intense competition around the innovative drugs. The United States, Israel, European Union, Australia, Italy, and Germany are the countries/locations developing fastest, with China showing progress in the discovery phase. Overall, the current competitive landscape of the target IL-1α and IL-1β is dynamic, with potential future developments in various countries and intense competition among different drug types.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
Conclusion
In summary, Rilonacept is a fusion protein drug developed by Regeneron Pharmaceuticals, Inc. It targets IL-1α and IL-1β, which is used in the treatment of various therapeutic areas including congenital disorders, skin and musculoskeletal diseases, immune system diseases, and cardiovascular diseases. The drug has been approved globally and received its first approval in the United States in 2008. It has been granted several regulatory designations, including priority review, overseas new drugs urgently needed in clinical settings, fast track, orphan drug, and breakthrough therapy.