Wave Life Sciences Declares First Submission of Clinical Trial Application for WVE-006, A Groundbreaking RNA Editing Clinical Candidate
Wave Life Sciences Ltd.,an advanced-stage RNA medicines corporation dedicated to providing transformative therapies for individuals grappling with devastating ailments, declared today its initial CTA submission for WVE-006 in cases of alpha-1 antitrypsin deficiency AATD.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
WVE-006, a novel, first-in-class RNA editing oligonucleotide incorporated with GalNAc, is engineered to rectify the solitary base mutation in mRNA coded by the SERPINA1Z allele. This action paves the way for the restoration and circulation of fully-functional, wild-type alpha-1 antitrypsin protein.
“The first CTA submission for WVE-006 marks the beginning of our clinical development journey for the world's initial RNA editing therapy," said Anne Marie Li-Kwai-Cheung, Wave Life Sciences' Chief Development Officer. "Our aim with WVE-006 is to correct the most recurrent genetic mutation responsible for AATD, offering an innovative treatment for those suffering from lung or liver diseases, or possibly both. We anticipate WVE-006 to usher in a transformative approach to AATD treatment.”
Wave Life Sciences' prospective clinical development plan for WVE-006 involves not only healthy subjects but also AATD individuals with the homozygous PiZZ mutation. This plan is aimed at producing a fast track to proof-of-mechanism, marked by the restoration of the M-AAT protein in the serum. By the last quarter of 2023, Wave plans to start dosing for healthy participants, aiming to generate proof-of-mechanism results in AATD patients by 2024.
Wave Life Sciences had begun its strategic partnership with GSK to progress groundbreaking RNA medicines using Wave’s cutting-edge multimodal RNA platform, inclusive of WVE-006. Wave benefitted from a $170 million upfront payment in cash and equity and is also receiving research funds. For WVE-006, Wave could earn up to $225 million in development and product launch milestone payments, along with up to $300 million in sales-based milestone remittances, and also tiered double-digit royalties based on net sales, peaking in the high teens.
Wave estimates its current cash resources should suffice for operations up to 2025. It should be noted, Wave's cash runway predictions do not include future milestones or contingent payments.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, organizations, clinical trials, clinical results, and drug patents related to this target.
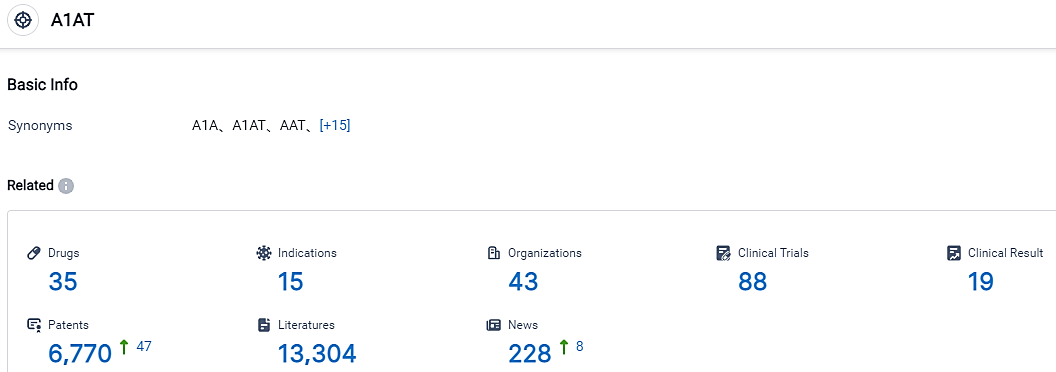
According to the data provided by the Synapse Database, As of September 7, 2023, there are 357 investigational drugs for the A1AT target, including 15 applicable indications,43 R&D institutions involved, with related clinical trials reaching 88,and as many as 6770 patents.
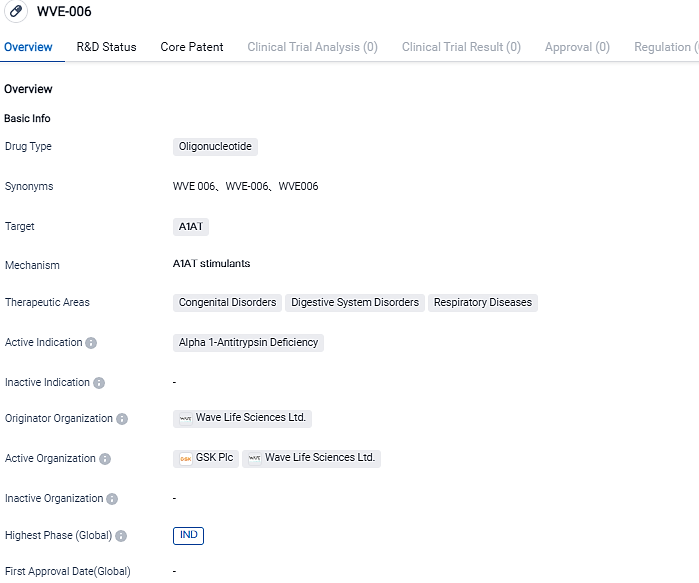
Wave Life Sciences Ltd. is in the process of developing an oligonucleotide medication, WVE-006, which is specifically designed to target A1AT. The drug is currently in the IND phase, indicating its advancement to clinical trials. Wave Life Sciences aims to provide a novel therapeutic option for patients with A1AT deficiency and related conditions in the field of biomedicine.