AceLink has unveiled the initial dosing of a patient in the Phase 2 clinical investigation of AL01211 for the treatment of Fabry Disease
AceLink Therapeutics, Inc., a clinical-phase biopharmaceutical enterprise advancing the forthcoming generation of oral substrate reduction treatments, has disclosed that the inaugural patient has commenced dosage of AL01211 in its Phase 2 study held in China targetting individuals afflicted with Fabry disease.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
AL01211 is a pioneering, orally administered inhibitor of glucosylceramide synthase that does not penetrate the brain and is being advanced to cure glycosphingolipid storage illnesses, such as Fabry Disease and Type 1 Gaucher Disease. In the first phase of clinical trials, the drug AL01211 appeared to be mostly well accepted with no severe side effects.
"Our team is thrilled to commence the process of participant enrollment in our Phase 2 investigation for individuals suffering from Fabry disease at one of China's top-ranked academic institutions," stated Pedro Huertas, M.D. Ph.D., AceLink Therapeutics' Chief Medical Officer.
"This accomplishment showcases the commitment, skill, and tireless efforts of our excellent team, along with the steadfast backing from our investigators. We hold the conviction that AL01211, as the top-rated GCS inhibitor, has the potential to confer significant remedial effects for glycosphingolipid metabolic disorders. We eagerly await to share developments as the research advances and persist in our resolve to expedite this prospective treatment closer to the patients who desperately require it," added Dr. Pedro Huertas.
The ongoing Phase 2 open-label investigation is assessing the safety profile, pharmacokinetics, pharmacodynamics, and therapeutic impacts of AL01211 in previously untreated male patients with classic Fabry disease. AceLink aims to enroll 18 patients across six locations in China. Dr. Nan Chen based at the Ruijin Hospital in Shanghai will lead the efforts as the Principal Investigator. AceLink anticipates top-line outcomes by second half of 2024.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
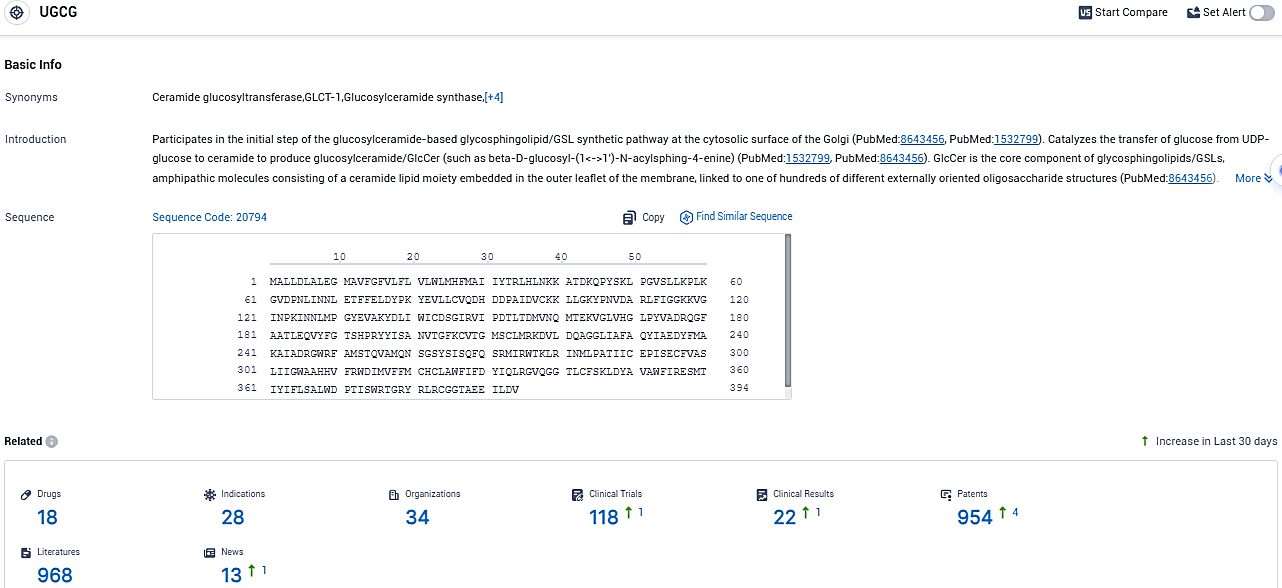
According to the data provided by the Synapse Database, As of November 3, 2023, there are 18 investigational drugs for the UGCG target, including 28 indications, 34 R&D institutions involved, with related clinical trials reaching 118, and as many as 968 patents.
AL01211 is a proprietary, non-brain penetrant UGCG inhibitor with excellent potency, great selectivity, and other favorable drug properties that support once-daily oral administration. AL01211 offers a much-needed oral small molecule therapy as an alternative to enzyme replacement therapy for Fabry disease obviating frequent intravenous infusions.