Lynparza and Imfinzi Surpass Chemotherapy in Advanced Endometrial Cancer Response
Recent evaluation of data from the phase III DUO-E clinical study reveals that combining Imfinzi (durvalumab) with platinum-regimen chemotherapeutics, and subsequently administering Imfinzi alongside Lynparza (olaparib), has enhanced performance across several important secondary efficacy measures. This combination was especially effective for individuals with advanced or recurring endometrial cancer that exhibits mismatch repair proficiency, when contrasted with the outcomes from using only chemotherapy.
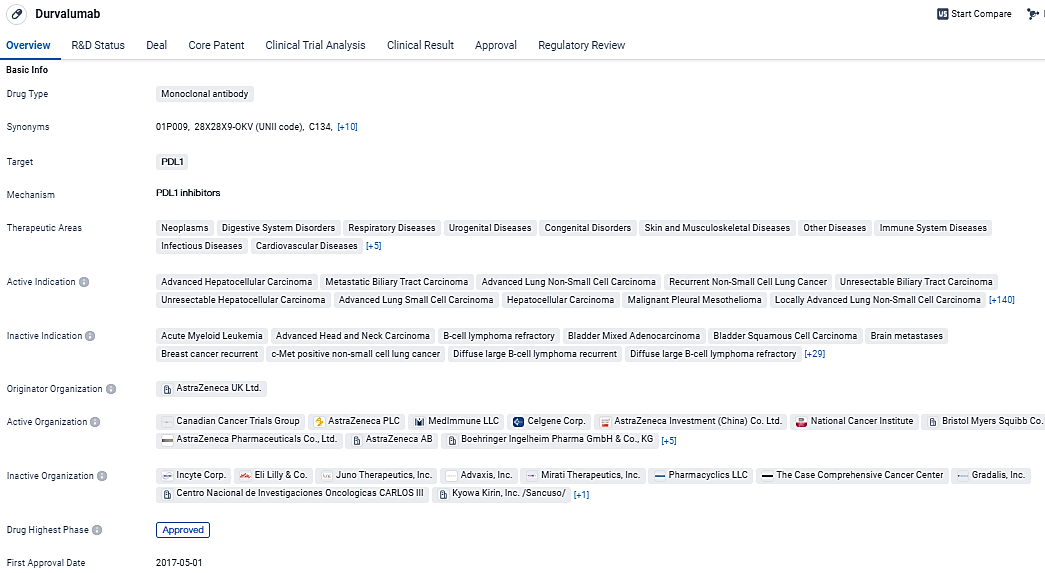
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
Today's late-breaking session at the Society of Gynecologic Oncology's 2024 Annual Meeting on Women’s Cancer in San Diego, California, will highlight key findings. A nuanced post-hoc subgroup examination was performed focusing on mismatch repair status, which is a significant biomarker in the study of endometrial cancer. According to the subgroup evaluation, the median response time for patients with proficient mismatch repair (pMMR) who received Lynparza and Imfinzi was over twice as long compared to those in the standard treatment group.
The research further revealed supporting secondary outcomes, underlining a consistent trend for pMMR subjects undergoing treatment with Lynparza and Imfinzi. This included a 32% decrease in the likelihood of experiencing either a second progression or mortality for those on the combination therapy relative to the standard regimen, along with an advancement in the interval to both the first and second additional therapies.
Across the entire cohort of the trial, participants receiving Lynparza and Imfinzi exhibited prolonged objective response rates (ORR) and duration of response (DoR). There was also a clear pattern of enhanced efficacy in secondary outcomes, such as overall survival, delay to the initial subsequent treatment, progression-free survival after second line therapy (PFS2), and time until the requirement of a second additional treatment.
Susan Galbraith, Executive Vice President of Oncology R&D at AstraZeneca, remarked, "The findings from the DUO-E trial indicate significant improvement in outcomes for patients with advanced endometrial cancer when Imfinzi is combined with standard chemotherapy. Additionally, for those who are pMMR and facing the most critical need, integrating Lynparza can potentiate the impact of checkpoint inhibitors in managing endometrial cancer.”
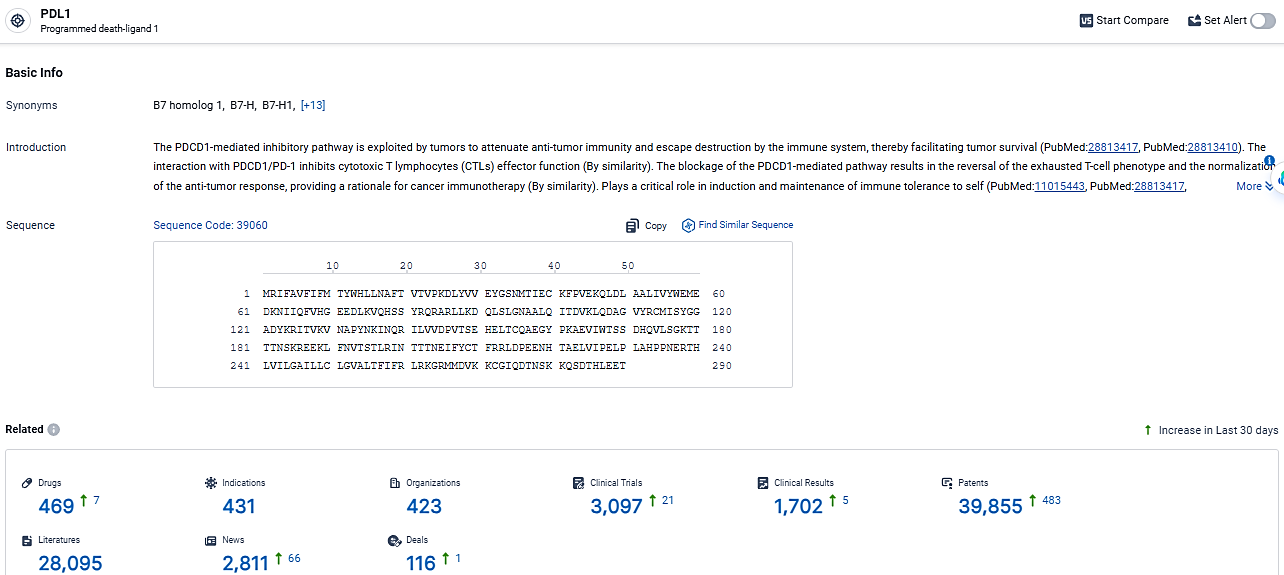
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of March 20, 2024, there are 469 investigational drugs for the PDL1 target, including 431 indications, 423 R&D institutions involved, with related clinical trials reaching 3097, and as many as 39855 patents.
Imfinzi (durvalumab) is a human monoclonal antibody that binds to the PD-L1 protein and blocks the interaction of PD-L1 with the PD-1 and CD80 proteins, countering the tumour’s immune-evading tactics and releasing the inhibition of immune responses. Durvalumab is a significant drug in the field of biomedicine, offering new treatment options for patients with various types of cancers. Its approval and designation as a breakthrough therapy highlight its potential to improve patient outcomes and address unmet medical needs.