Adcentrx Therapeutics Gains FDA Approval for Novel ADC, ADRX-0405 Targeting STEAP1
Adcentrx Therapeutics, a biotechnology firm in the clinical development phase that focuses on advancing novel protein conjugates for cancer and other severe illnesses, has declared that the U.S. Food and Drug Administration (FDA) has approved the company's Investigational New Drug (IND) application for ADRX-0405 aimed at treating specific advanced solid tumors.
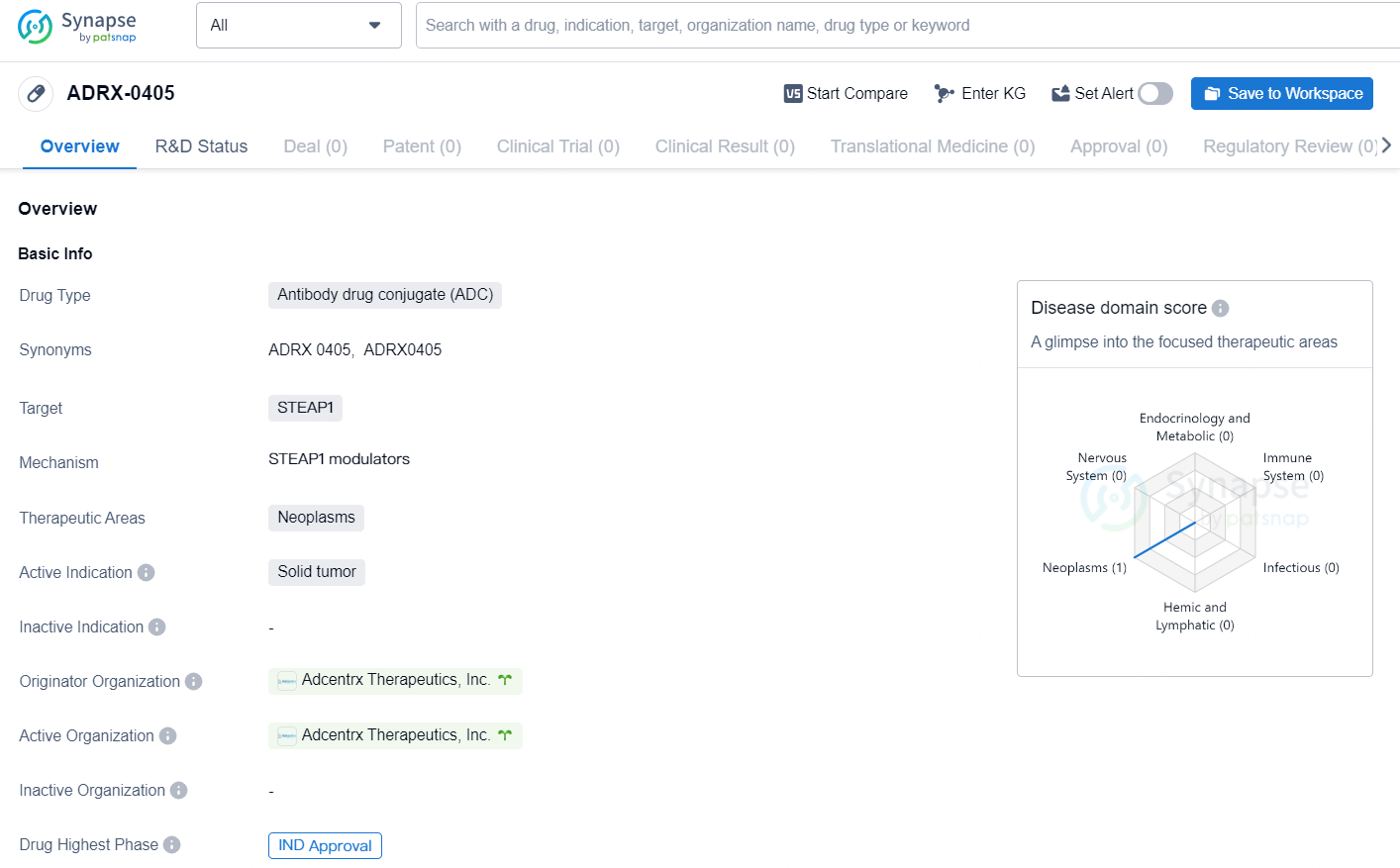
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
ADRX-0405 is a novel antibody-drug conjugate (ADC) that features a humanized IgG1 antibody targeting the six-transmembrane epithelial antigen of the prostate 1 (STEAP1), a surface protein that is often overexpressed in prostate cancer and some other malignancies, while being minimally present in normal tissues. The approval of our second Investigational New Drug (IND) application by the FDA represents a significant achievement for Adcentrx.
The proprietary i-Conjugation® technology platform developed by Adcentrx plays a crucial role in the formulation of ADRX-0405. This platform incorporates protease-cleavable linkers and stable conjugation chemistry, which improve the delivery of the therapeutic agent. This sophisticated technology guarantees a highly stable ADC with a topoisomerase inhibitor payload linked at a drug-to-antibody ratio of eight (DAR 8). Preclinical evaluations of ADRX-0405 have shown promising pharmacokinetics, safety profile, and considerable effectiveness across various tumor models.
"The FDA's approval of our second IND represents another key step forward for Adcentrx," remarked Hui Li, Ph.D., President and CEO of the company. "We are thrilled about the first-in-class potential of ADRX-0405 and the chance to significantly impact patients facing advanced cancers, particularly those with metastatic castration-resistant prostate cancer, who are in critical need of new targeted treatment options."
The upcoming Phase 1a/b clinical trial of ADRX-0405 will be an open-label, multicenter, non-randomized study focusing on dose escalation and expansion. This trial will recruit patients with specific advanced solid tumors, including metastatic castration-resistant prostate cancer. The primary goals of the trial will include assessing the safety and tolerability of ADRX-0405 and identifying the optimal dosage. The company plans to begin enrolling the first patient in the fourth quarter of 2024, with initial data expected to be available in the fourth quarter of 2025.
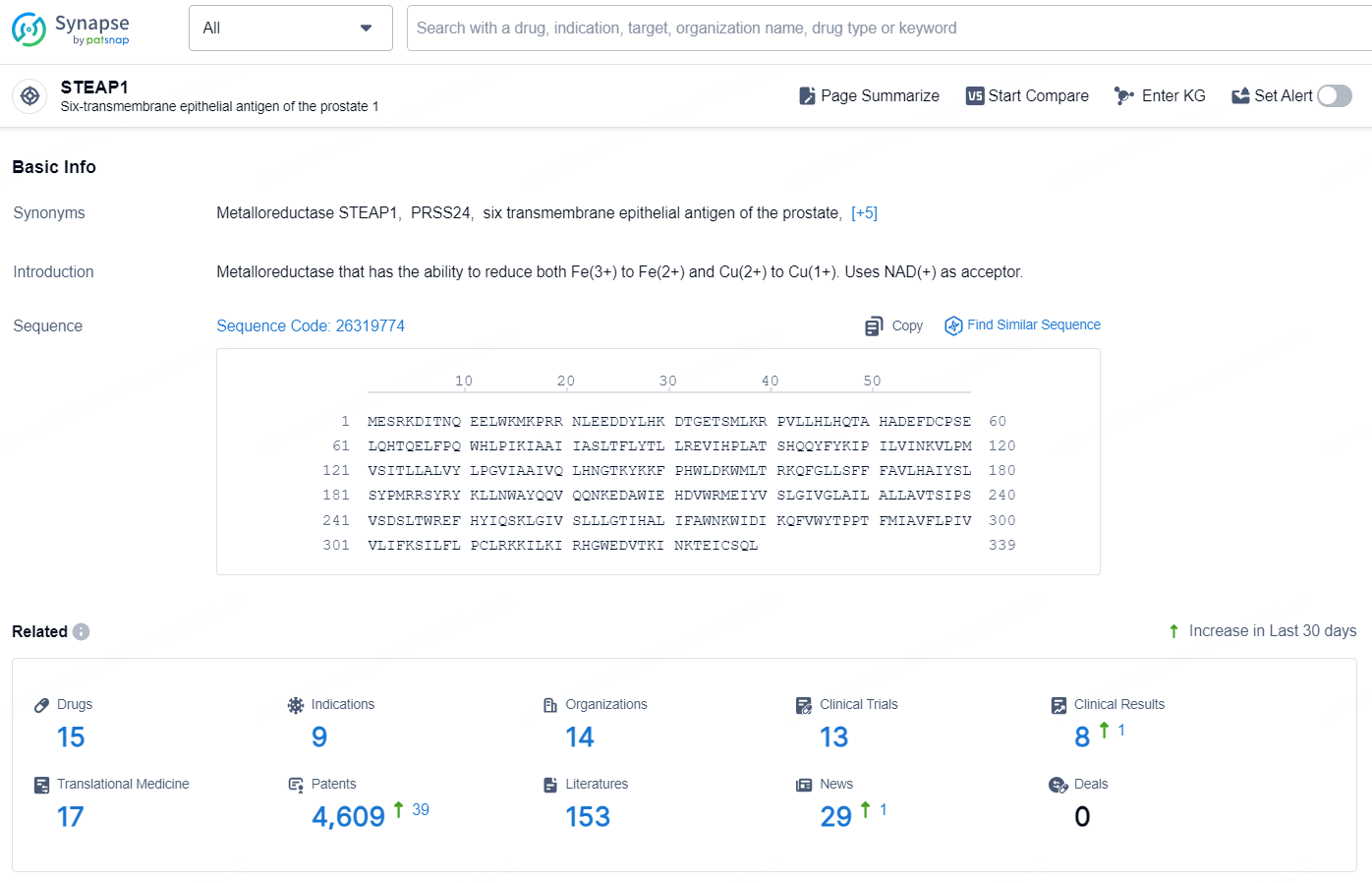
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Chemical, As of October 21, 2024, there are 15 investigational drugs for the STEAP1 target, including 9 indications, 14 R&D institutions involved, with related clinical trials reaching 13, and as many as 4609 patents.
ADRX-0405 is an antibody drug conjugate (ADC) developed by Adcentrx Therapeutics, Inc. The drug targets STEAP1 and is intended for the treatment of neoplasms, specifically solid tumors. As of the latest available information, ADRX-0405 has received IND (Investigational New Drug) approval, indicating that it has cleared the initial regulatory hurdles necessary to proceed with clinical trials.