AstraZeneca Submits Application for "Dual Immunotherapy Combination" in China
On October 18, 2024, according to the CDE website, AstraZeneca submitted a market application for the injectable drug Tremelimumab. This drug has already been approved overseas for use in combination with a PD-L1 antibody for the first-line treatment of hepatocellular carcinoma and non-small cell lung cancer.
In this submission, the company also filed for the approval of Durvalumab, suggesting that the two drugs could be used in combination as a first-line treatment for tumors.
About Tremelimumab
Tremelimumab is a first-generation CTLA-4 antibody that was initially developed by Pfizer, entering Phase III clinical trials even before the first approved CTLA-4 antibody, Ipilimumab. In 2008, Pfizer abandoned Tremelimumab after interim analyses showed no difference in overall survival (OS), eventually transferring the product to MedImmune in 2011 (MedImmune was acquired by AstraZeneca in 2007). Subsequent attempts to use Tremelimumab as monotherapy were unsuccessful, making its combination with a PD-1 antibody the last hope for its market approval.
In the fall of 2019, AstraZeneca disclosed details of the POSEIDON trial. In this trial, Imfinzi (Durvalumab) combined with platinum-based chemotherapy, or a combination of Imfinzi, Tremelimumab, and chemotherapy, was compared to chemotherapy alone for first-line treatment of Stage IV metastatic NSCLC. Tremelimumab significantly improved progression-free survival (PFS), although the interim analysis of the POSEIDON trial did not indicate sufficient advantages at that time. However, in May 2021, final high-level positive results showed that the combination of Imfinzi, Tremelimumab, and chemotherapy offered statistically and clinically significant OS benefits over chemotherapy alone. Each combination demonstrated acceptable safety profiles with no new safety signals detected.
Statistics show that the combination of Tremelimumab and Durvalumab has been approved for hepatocellular carcinoma and non-small cell lung cancer. Beyond these indications, the company is also exploring their use in locally advanced hepatocellular carcinoma (EMERALD-3 study), small cell lung cancer (ADRIATIC study), bladder cancer (VOLGA and NILE studies), and other indications.
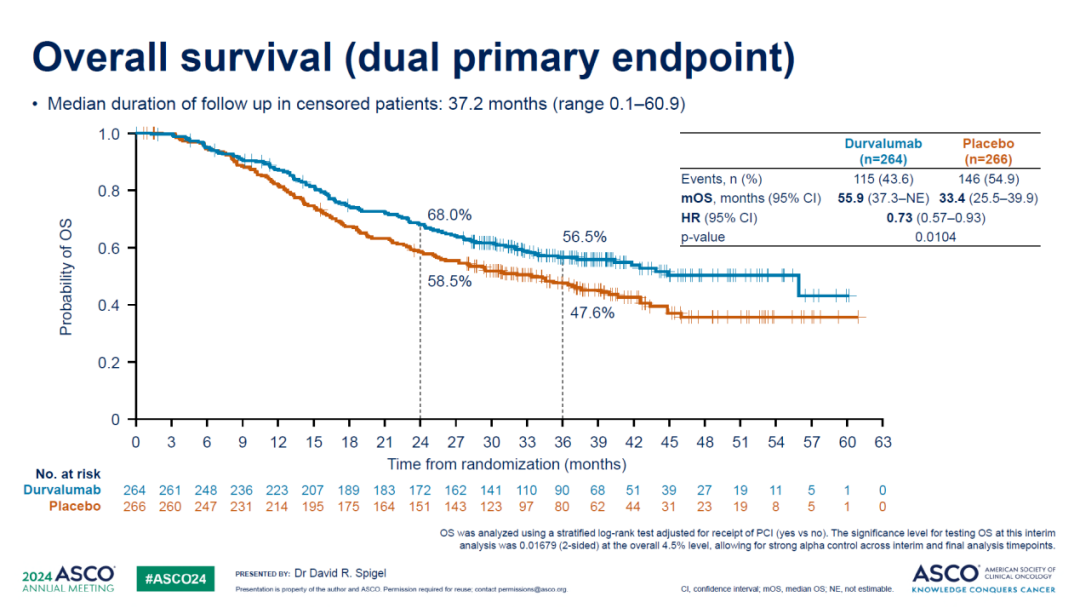
In September 2024, the company released significant 5-year OS data from the HIMALAYA study. The HIMALAYA study is a randomized, open-label, multi-center, global Phase III trial with a primary endpoint of OS in the intent-to-treat population comparing the STRIDE regimen to Sorafenib. The results showed an unprecedented 5-year OS rate of 19.6% for the STRIDE regimen group, double that of the Sorafenib group, reinforcing its status in first-line treatment for liver cancer.
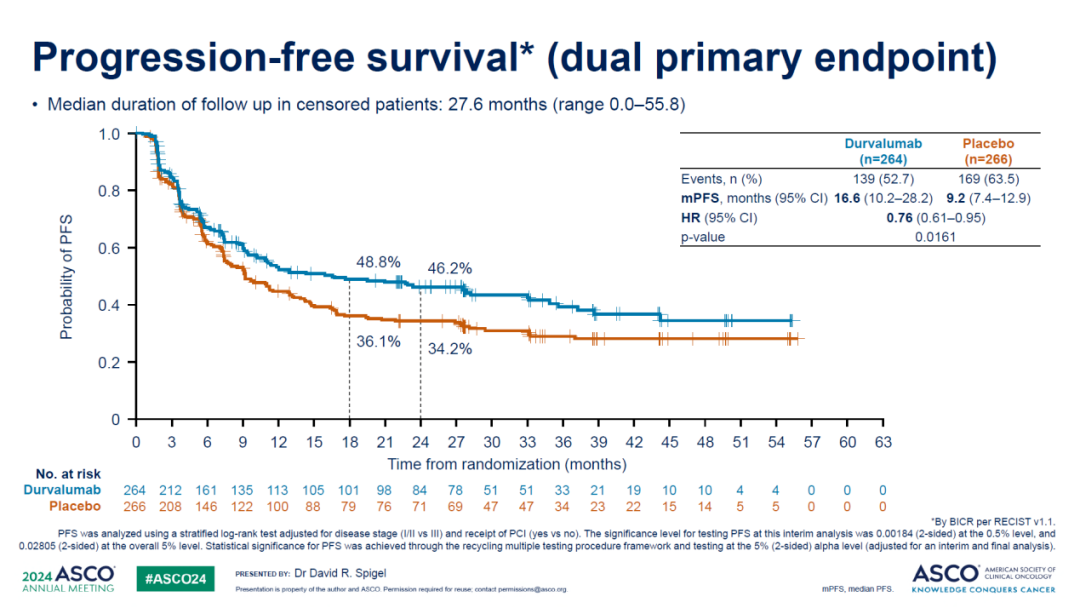
Furthermore, ADRIATIC is the first global Phase III immunotherapy trial targeting LS-SCLC, which reached both PFS and OS primary endpoints this April. The study aimed to evaluate the efficacy of Durvalumab ± Tremelimumab versus placebo in 730 LS-SCLC patients who had not progressed after cCRT. This clinical study was conducted across 164 research centers in 19 countries in North America, South America, Europe, and Asia.
About CTLA-4 Antibodies
CTLA-4, also known as CD152, is a transmembrane protein found on the surface of T cells and is encoded by the CTLA-4 gene, located at chromosome 2q33. It is primarily expressed on the surface of activated T lymphocytes. CTLA-4 shares significant similarities with CD28, a co-stimulatory receptor molecule on T cells, in terms of gene structure, chromosomal positioning, sequence homology, and gene expression. CTLA-4 can bind to co-stimulatory molecules (B7) on the surface of antigen-presenting cells (APCs).
CTLA-4 exists as a homodimer composed of an extracellular domain, a transmembrane region, and a cytoplasmic region. It is mainly expressed on activated CD4+ and CD8+ T cells and also found on the surface of Treg cells. The CTLA-4 expressed on Tregs plays a crucial role in their function, accumulating within tumor tissues to suppress anti-tumor immunity. By blocking CTLA-4, the function of tumor-infiltrating Tregs is mitigated, thereby enhancing immune responses within the tumor. Consequently, CTLA-4 is considered an immunosuppressive molecule that hinders anti-tumor immunity, making it a focal point in current tumor-targeted immunotherapy.
According to incomplete statistics, there are about a hundred CTLA-4 therapies under investigation globally, with seven products already approved. Building on the considerable success of combination therapies, companies like Hengrui, Akeso, Innovent, and Henlius in China are actively exploring related combination treatment strategies.
Selection of Drugs for Liver Cancer
As a country with a high prevalence of liver cancer, China accounts for nearly half of the world's new and fatal cases each year. Currently, both domestic and international clinical studies have been extensively conducted on first-line immunotherapy for advanced hepatocellular carcinoma (HCC). Notably, the "Double A" regimen, the "T+A" regimen, and the "Double D" regimen have all received approval from the National Medical Products Administration (NMPA) for marketing and have been included as a Level I recommendation (Category 1A evidence) in the "CSCO Guidelines for the Diagnosis and Treatment of Primary Liver Cancer."
In addition to combination therapies involving targeted and immune approaches, the NMPA has also approved toripalimab as a monotherapy for the first-line treatment of patients with advanced HCC. Furthermore, the HIMALAYA study yielded positive results for the combination of tremelimumab and durvalumab, although this dual immunotherapy regimen has not yet been approved domestically.
Currently, several studies focusing on immunotherapy combined with local treatments are underway, promising to offer more therapeutic options for liver cancer patients in the near future.
How to obtain the latest research advancements in the field of biopharmaceuticals?
In the Synapse database, you can keep abreast of the latest research and development advances in drugs, targets, indications, organizations, etc., anywhere and anytime, on a daily or weekly basis. Click on the image below to embark on a brand new journey of drug discovery!