FDA Approves BOTOX® Cosmetic for Moderate to Severe Neck Bands
Allergan Aesthetics, a subsidiary of AbbVie (NYSE: ABBV), has received approval from the U.S. FDA for BOTOX® Cosmetic. This treatment is authorized for the temporary enhancement of moderate to severe vertical platysma bands that link the jaw and neck in adult patients. BOTOX® Cosmetic is unique as it is the sole product approved for four aesthetic treatment areas: forehead lines, frown lines, crow’s feet, and now platysma bands, marking it as the pioneering product that extends its application beyond facial areas.
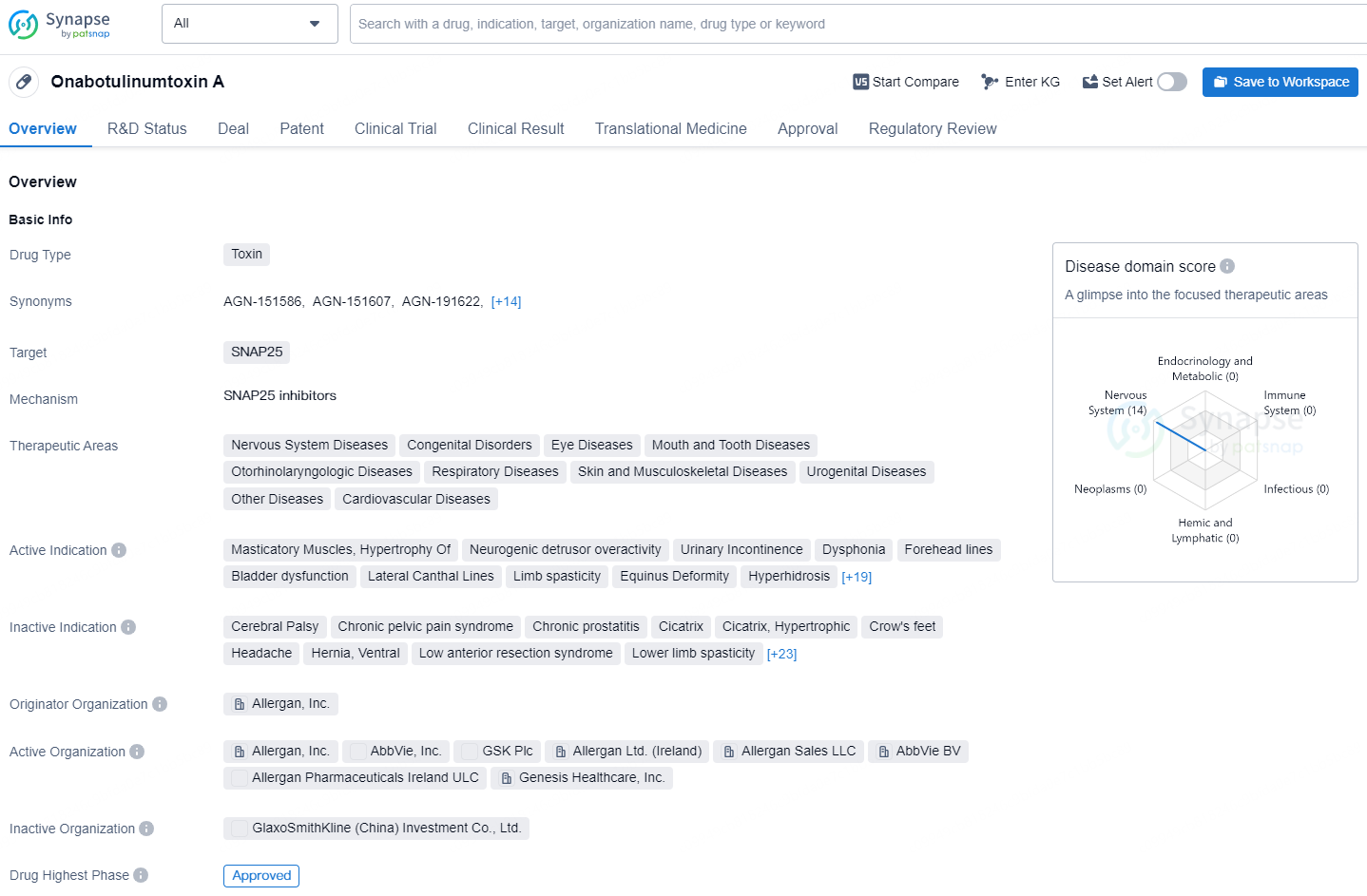
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
Research indicates that a significant number of consumers in the U.S. find their platysma bands to be a major concern. With the recent approval, there is now a nonsurgical injectable solution available that can temporarily enhance the appearance of the vertical bands that connect the neck and jaw, stated Darin J. Messina, Ph.D., Senior Vice President of Aesthetics R&D at Allergan Aesthetics. “This new indication for BOTOX® Cosmetic marks a true advancement in the field. We are thrilled to provide new opportunities for both patients and providers in reaching their aesthetic aspirations.”
The platysma is a superficial muscle that spans across the neck and lower portion of the face. When this muscle contracts, it may result in noticeable bands on the neck, potentially leading to a less distinct jawline. The use of BOTOX® Cosmetic targets the muscle activity below the skin, which temporarily alleviates the appearance of these bands that extend from the jaw to the neck. By administering BOTOX® Cosmetic along the jawline and the vertical bands using an FDA-approved dosage—26, 31, or 36 units depending on the severity—patients can experience a temporary reduction in muscle activity. Individuals are advised to consult with their licensed aesthetic professional to evaluate whether this treatment is suitable for them.
“In my practice, I always include the neck and lower face during aesthetic evaluations. Many patients are often surprised by how significant changes in these regions can influence their overall appearance,” remarked Dr. Terrence Keaney, a board-certified dermatologist and key investigator in clinical trials. “With the recent FDA approval of BOTOX® Cosmetic for addressing platysma bands, coupled with specific injection techniques and dosing, I can now reliably present my patients with a treatment that may achieve the outcomes they desire.”
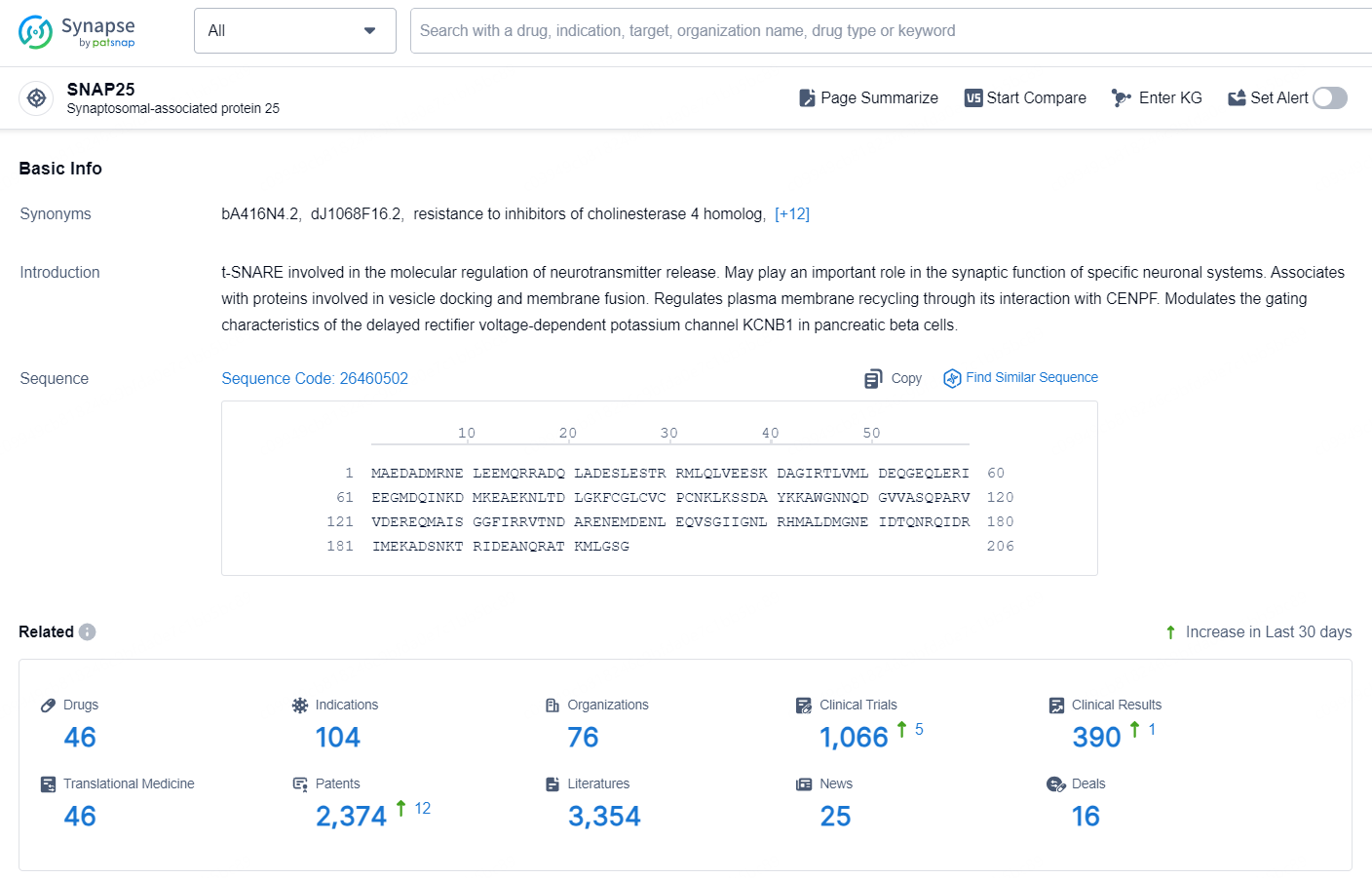
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of October 23, 2024, there are 35 investigational drugs for the SNAP25 targets, including 26 indications, 50 R&D institutions involved, with related clinical trials reaching 137, and as many as 9179 patents.
Onabotulinumtoxin A is a toxin drug that targets SNAP25 and is used in the treatment of various therapeutic areas, including nervous system diseases, congenital disorders, eye diseases, mouth and tooth diseases, otorhinolaryngologic diseases, respiratory diseases, skin and musculoskeletal diseases, urogenital diseases, cardiovascular diseases, and other diseases.