Advancements and Global Market Trends in Stem Cell Therapy
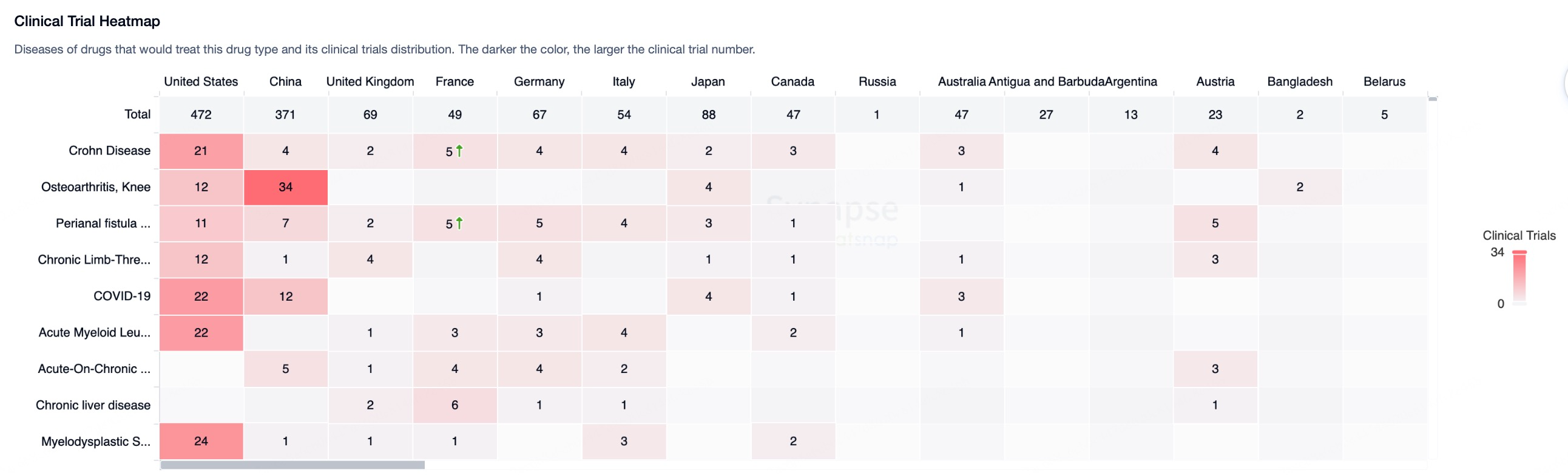
The global stem cell therapy market was valued at $10–12 billion in 2022 and is projected to grow at a compound annual growth rate (CAGR) of 10–15%, potentially surpassing $31.41 billion by 2030. The application prospects for stem cell therapies can be categorized into three main areas: off-the-shelf cell therapies, regenerative medicine, and gene therapy vehicles. Among the 25 approved stem cell therapies worldwide, mesenchymal stem cell (MSC) therapies and hematopoietic stem cell (HSC) therapies constitute 76% of the total, reflecting their advanced technological maturity.
Mechanisms of Stem Cell Therapy
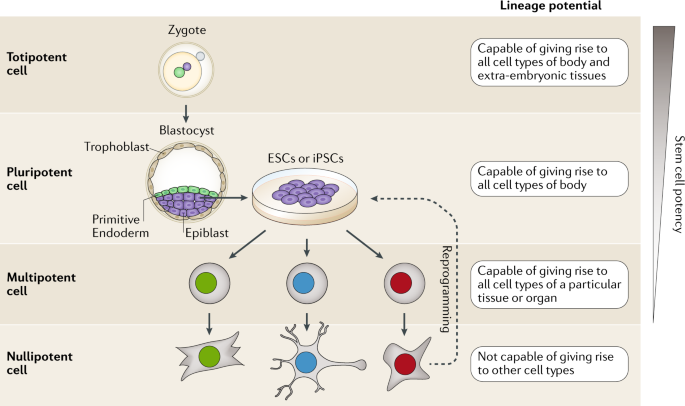
The mechanisms of stem cell therapy revolve around their core properties of self-renewal and multi-lineage differentiation, which grant them significant value in regenerative medicine and tissue repair. Self-renewal refers to the ability of stem cells to proliferate indefinitely without differentiation, maintaining their undifferentiated state. Through mitosis, stem cells produce progeny that preserve stem cell quantity and functionality. This process is regulated by multiple signaling pathways, including the transcription factors Oct4, Sox2, and Nanog in embryonic stem cells, while adult stem cells rely primarily on the p16-Rb and p19-p53 signaling pathways.
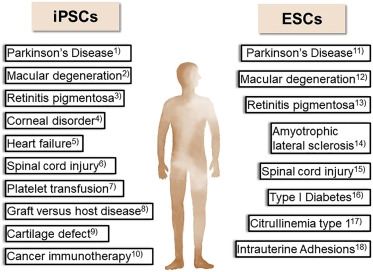
Multi-lineage differentiation allows stem cells to transform into various cell types, such as neurons, cardiomyocytes, and osteocytes, under specific conditions. This differentiation process is influenced by microenvironmental factors and signaling pathways like Wnt, Notch, BMP, and Hedgehog. By introducing specific growth factors and chemical agents, scientists can precisely guide stem cell differentiation into desired cell types for treating diseases such as osteoarthritis, spinal cord injuries, myocardial infarction, and neurological disorders.
Stem cells also exhibit remarkable immunomodulatory and anti-inflammatory effects, helping to suppress abnormal immune responses and restore immune system balance. MSCs, in particular, have been widely studied and proven effective in these areas. MSCs regulate immune cell function through direct cell-to-cell contact and secretion of effector molecules such as growth factors, cytokines, and chemokines. For instance, MSCs can inhibit T-cell activation and proliferation while promoting the activity of regulatory T cells (Tregs), thereby alleviating inflammation. Additionally, MSCs secrete anti-inflammatory factors like transforming growth factor-beta (TGF-β) and interleukin-10 (IL-10), which reduce inflammatory mediators, alleviate symptoms, and protect tissues from further damage.
The paracrine effects of stem cells involve the secretion of various bioactive molecules such as cytokines, growth factors, chemokines, microRNAs, and extracellular vesicles, which participate in intercellular communication, tissue repair, and regeneration. These molecules play crucial roles in diverse physiological and pathological processes. For example, growth factors secreted by stem cells, including vascular endothelial growth factor (VEGF), fibroblast growth factor (FGF), and platelet-derived growth factor (PDGF), promote angiogenesis, accelerate wound healing, and facilitate tissue repair. Anti-inflammatory cytokines such as interleukin-6 (IL-6), IL-10, and TGF-β help regulate immune cell function, suppress inflammation, and support tissue repair. For instance, IL-10 reduces the production of inflammatory mediators, mitigating inflammation and protecting tissues from further damage.
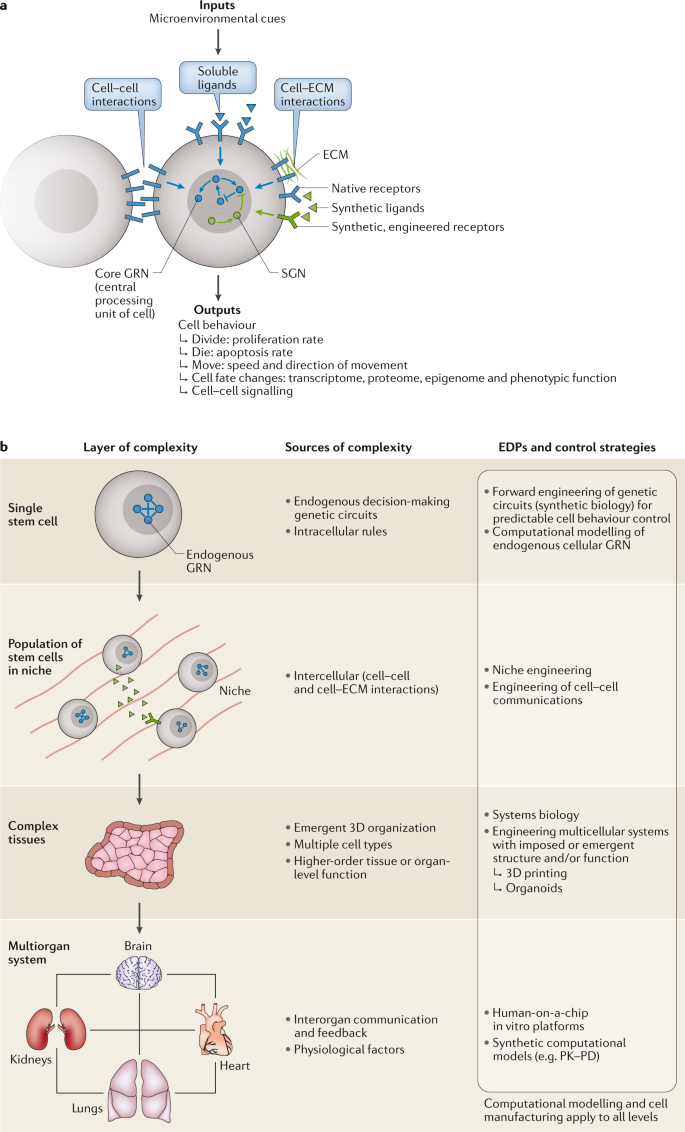
Stem cells possess homing ability, a critical biological characteristic that enables them to migrate toward injured or inflamed sites in the body. Damaged tissues release bioactive factors that bind to receptors on the surface of stem cells, directing them to the injury site. For example, damaged tissues secrete stromal cell-derived factor-1 (SDF-1), which interacts with the CXCR4 receptor on stem cells, guiding their migration. Additionally, growth factors, adhesion molecules, and inflammatory factors released at the injury site further enhance stem cell migration and homing. The homing process encompasses not only migration but also the differentiation of stem cells into necessary cell types within the specific microenvironment of the injury, contributing to tissue repair and regeneration.
Stem cells can differentiate into various types of tissue cells, aiding the regeneration and repair of damaged or dysfunctional tissues. Whether addressing skin wounds, myocardial injury, or neurodegenerative diseases, stem cell therapy provides new cellular sources for damaged tissues, promoting recovery and functional reconstruction. For instance, mesenchymal stem cells (MSCs) can differentiate into bone, cartilage, and adipose cells, making them suitable for treating conditions like osteoarthritis and spinal cord injuries. Their multi-lineage differentiation potential allows stem cells to transform into cell types needed by injured tissues under specific conditions, replacing damaged or dead cells and restoring tissue function. For example, following myocardial infarction, MSCs can differentiate into cardiomyocytes, replacing damaged myocardial cells and improving cardiac function. This cell replacement capability not only offers a new source of cells but also facilitates structural and functional recovery of damaged tissues.
Another critical mechanism is microenvironment regulation. Stem cells secrete various growth factors and cytokines to modulate the microenvironment of injured tissues, promoting cell proliferation and tissue repair. For example, hepatocyte growth factor (HGF) secreted by stem cells supports liver cell regeneration and improves liver function. Additionally, vascular endothelial growth factor (VEGF) promotes angiogenesis, improving blood supply to damaged tissues and accelerating the repair process.
Stem cells can also secrete anti-apoptotic factors that prevent cell death, which is crucial for protecting damaged tissues and maintaining tissue homeostasis. By inhibiting apoptosis, stem cell therapy enhances tissue survival and repair, improving therapeutic outcomes. Anti-apoptotic factors such as Bcl-2 and Bcl-xL inhibit apoptotic signaling pathways, protecting cells from damage. For instance, Bcl-2 prevents apoptosis by inhibiting the release of cytochrome c from mitochondria, thereby protecting cells in damaged tissues. This anti-apoptotic effect helps maintain tissue integrity and functionality, reducing cell loss. Additionally, stem cells secrete antioxidants and anti-inflammatory factors, shielding cells from oxidative stress and inflammatory damage, and preserving cell survival and function.
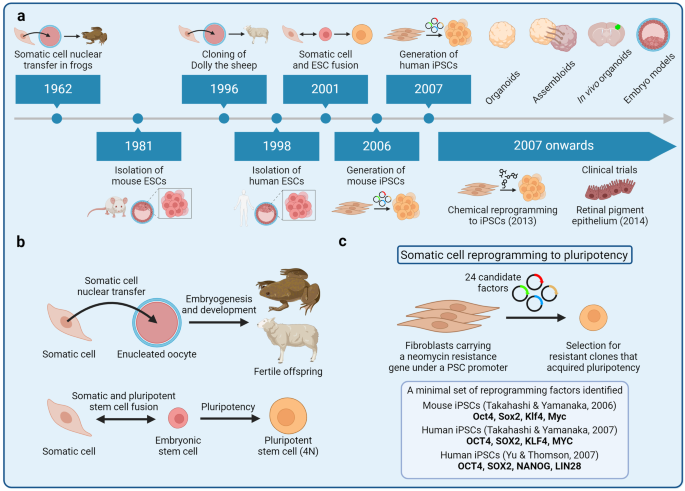
Regional R&D Characteristics of Stem Cell Therapy
As a regenerative medicine technique, stem cell therapy uses stem cells or their derivatives to stimulate the body's healing processes and repair damaged, diseased, or injured tissues, representing a promising frontier in transplantation.
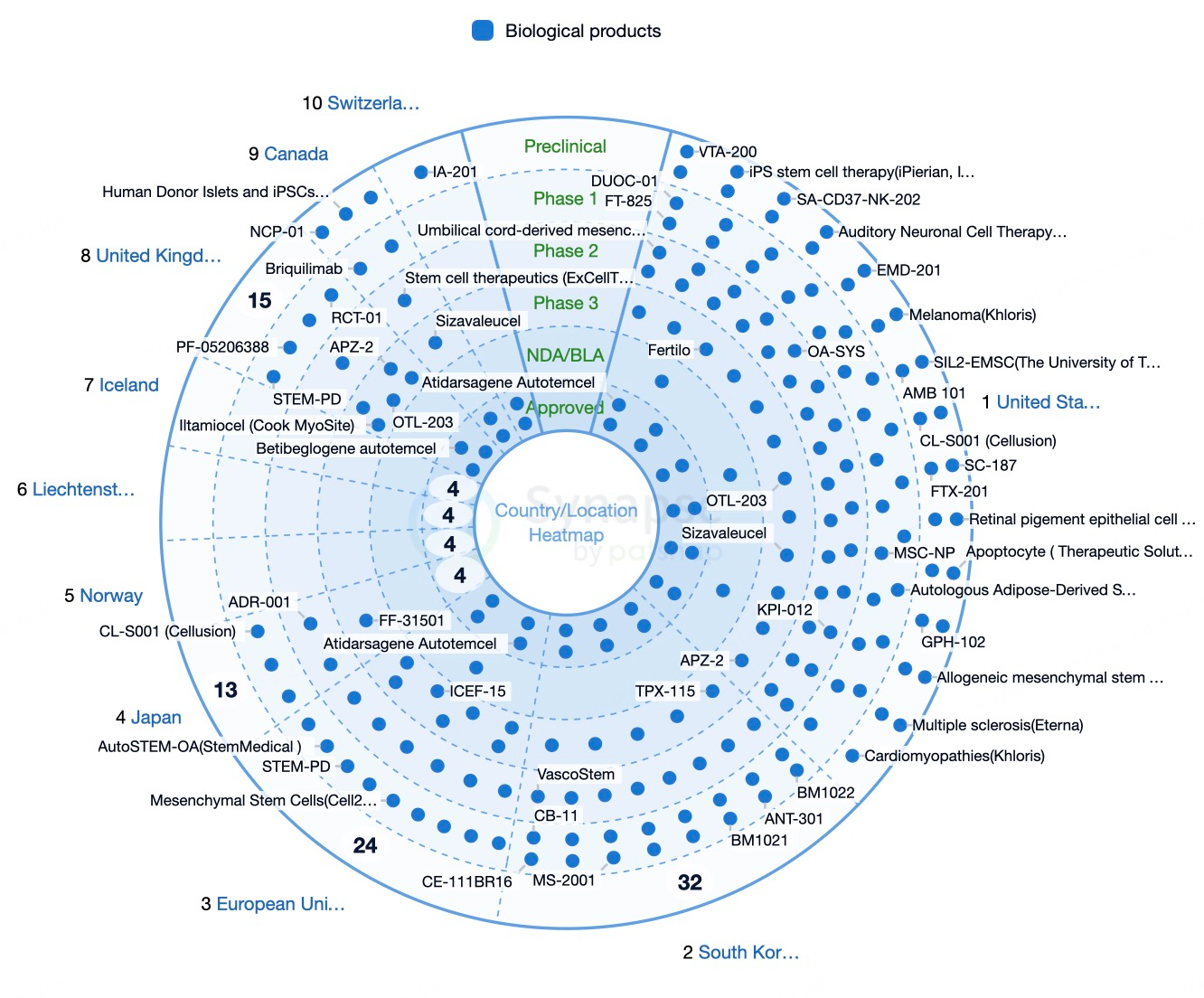
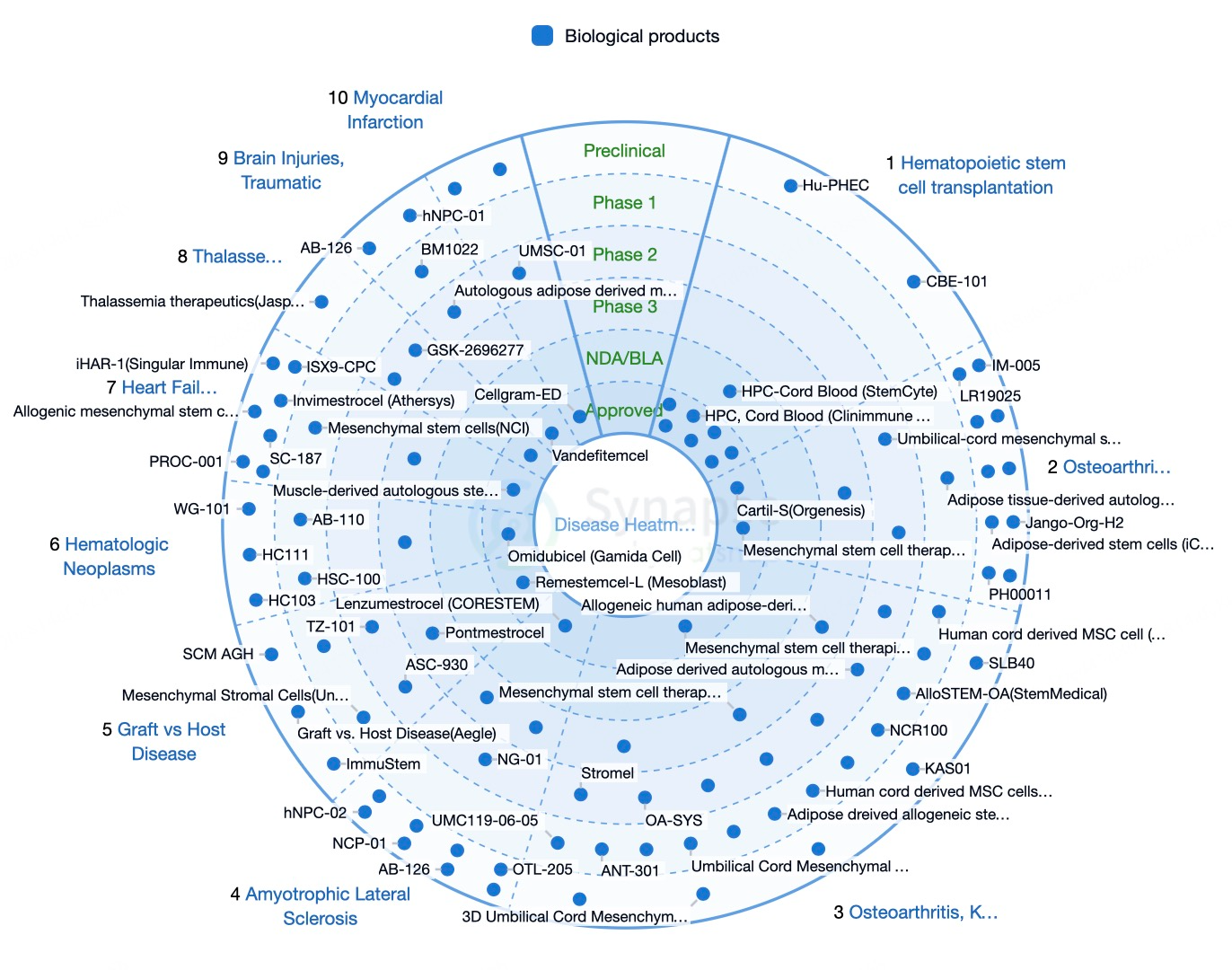
The United States leads stem cell research and application, supported by a robust scientific foundation and a comprehensive regulatory framework. Hematopoietic stem cell transplantation for hematologic diseases was among the earliest stem cell technologies adopted clinically and remains the only globally recognized stem cell therapy. U.S. research institutions and companies, such as the Mayo Clinic, have made significant advances, exploring stem cell treatments for neurodegenerative diseases, cardiac conditions, and diabetes.
Europe is also at the forefront, with countries like Germany, the UK, and France making notable contributions through institutions and companies like BioTime and STEMCELL Technologies. Germany's Max Planck Institute and the University of Cambridge in the UK have achieved significant breakthroughs in both fundamental and clinical applications of stem cells. European firms like TiGenix and Promethera Biosciences have conducted extensive clinical trials on stem cell therapies for liver and cardiovascular diseases.
China has made remarkable progress in stem cell research and applications, fostered by governmental support and favorable policies. Institutions like Peking University and companies affiliated with East Hospital have achieved notable outcomes. Japan, led by Kyoto University and the University of Tokyo, has also made significant advances, with Shinya Yamanaka receiving a Nobel Prize for discovering induced pluripotent stem cells (iPSCs). South Korean firms such as Celltrion and GC Pharma have conducted extensive trials on stem cell therapies for autoimmune and neurodegenerative diseases. Indian companies, like Stempeutics Research, have also made strides in treating osteoarthritis and spinal cord injuries using stem cell therapies.
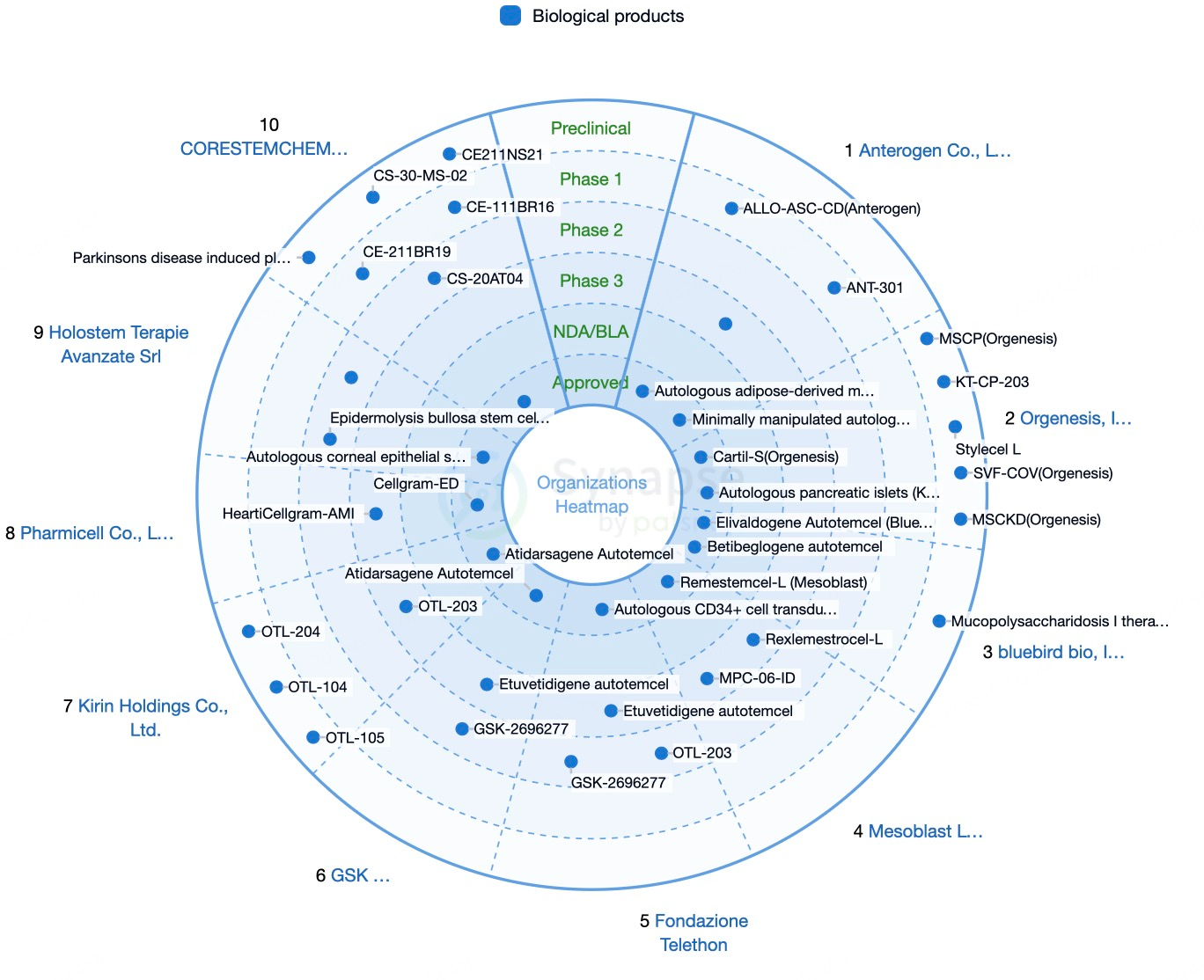
Global Competitive Landscape of Stem Cell Therapy
Fate Therapeutics is a leading biopharmaceutical company specializing in regenerative medicine and immunotherapy, focusing on developing immune cell therapies based on induced pluripotent stem cells (iPSCs). Utilizing its proprietary iPSC technology platform, the company generates and expands NK and T cells with specific biological properties for treating cancer and other immune-related diseases. Key products include iPSC-derived NK cells (such as FT500, FT516, FT538, and FT596) and T cells (such as FT819), which have demonstrated good safety profiles and preliminary efficacy in various clinical trials. For example, in a Phase 1 clinical trial for relapsed/refractory B-cell lymphoma, FT516 achieved complete remission in six out of eight patients. Fate Therapeutics has a strong foundation in iPSC technology, a broad patent portfolio, and collaborations with major pharmaceutical companies, accelerating its product development and commercialization. Its robust manufacturing capabilities and extensive clinical data position it as a leader in stem cell drug research.
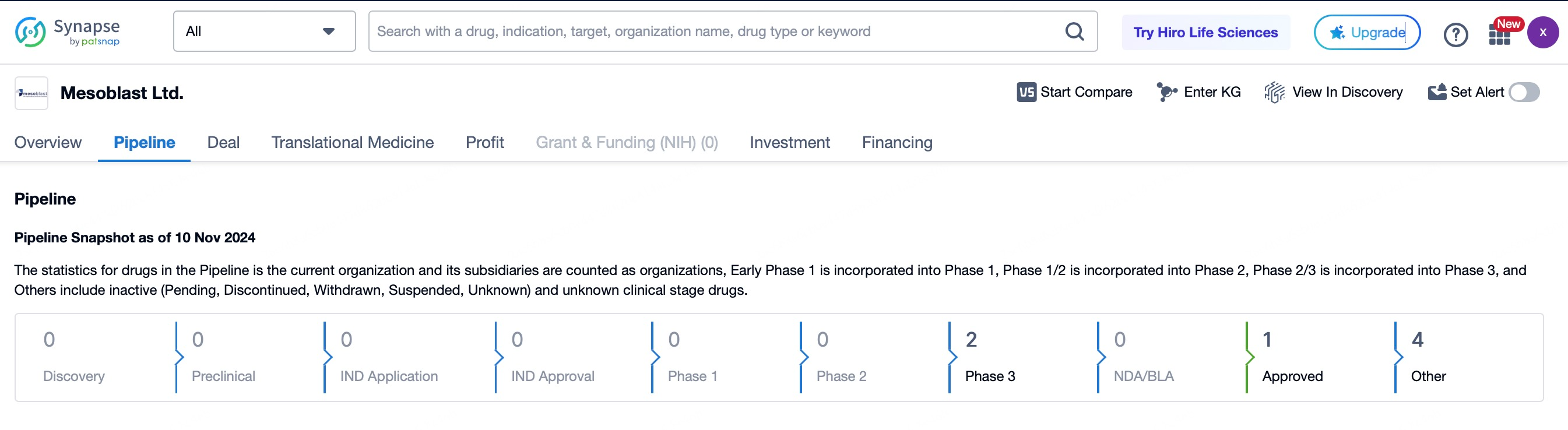
Mesoblast Limited is a biopharmaceutical company dedicated to developing allogeneic mesenchymal precursor cell (MPC)-based therapeutic products, focusing on cardiovascular, orthopedic, and immune diseases. Its flagship product, Remestemcel-L, has been approved in Japan for treating steroid-refractory acute graft-versus-host disease (SR-aGVHD) and has shown promising results in clinical trials for chronic heart failure and acute respiratory distress syndrome (ARDS). Another key product, Rexlemestrocel-L, has exhibited significant efficacy in Phase 3 trials for chronic heart failure and chronic low back pain. With its proprietary MPC technology platform and extensive clinical experience, Mesoblast has amassed substantial clinical data in the cardiovascular and orthopedic fields, showcasing its strong competitive edge. The company’s partnerships with global pharmaceutical firms have further accelerated its product development and commercialization.
Athersys, Inc. is a biotechnology company focused on regenerative medicine, primarily developing MultiStem® cell therapy based on multipotent adult progenitor cells (MAPCs). This therapy has shown broad therapeutic potential and positive clinical results for diseases such as stroke, traumatic brain injury, and ARDS. MultiStem® has received FDA Fast Track and RMAT designations, expediting its development and approval processes. Leveraging its proprietary technology platform and rich clinical experience, Athersys collaborates with international partners to advance the clinical research and commercialization of its products, offering new treatment options for patients with various diseases.
BlueRock Therapeutics focuses on cell therapies using iPSC technology, targeting neurodegenerative and cardiovascular diseases. Its core product, bemdaneprocel (BRT-DA01), an iPSC-derived dopaminergic neuron therapy for Parkinson’s disease, has received FDA Fast Track designation and demonstrated good safety and cell survival in Phase 1 clinical trials. BlueRock benefits from its close collaboration with Bayer, which provides strong R&D and financial support, positioning it as a leader in the cell therapy field.
TiGenix NV (acquired by Takeda) focuses on gastrointestinal and cardiovascular diseases, with its primary product, Cx601 (allogeneic adipose-derived stem cells), approved in the EU as the first stem cell therapy for complex perianal fistulas. Promethera Biosciences specializes in liver diseases, with its main product, HepaStem® (allogeneic liver stem cells), targeting acute liver failure and supported by unique technology and multiple clinical trials. Cellular Biomedicine Group (CBMG) focuses on cancer immunotherapy and autoimmune diseases, leveraging its experience with CAR-T and mesenchymal stem cell therapies, with several products in clinical trial stages.
In China, significant progress has been made in the stem cell drug field. EdiGene focuses on gene editing and stem cell therapy, with its edited hematopoietic stem cell product ET-01 approved for clinical trials, showcasing deep expertise in gene editing technology. BGI Shenzhen excels in genomics and stem cell research, offering personalized and precision medicine solutions widely applied in genetic disease diagnosis and cancer screening. Vcanbio dominates China’s cell storage market, providing high-quality cell storage services and stem cell therapy products, with several stem cell products, such as dental pulp stem cell injections and Inaticabtagene Autoleucel cell injections, entering clinical trials. Guanhao Biotech has extensive experience in regenerative medicine and cell therapy, offering various biomaterials and cell therapy products, with several advancing to clinical trials. These companies highlight China’s progress and innovation in the stem cell drug field. With technological advancements and policy support, China is expected to achieve more breakthroughs, offering global patients expanded treatment options.
Summary
The global stem cell therapy market is projected to grow steadily, potentially exceeding $31.41 billion by 2030. The application of stem cell therapy continues to expand from hematologic diseases to neurological, cardiovascular, and metabolic disorders. With improved policies, continuous innovation, and deeper clinical applications, China's stem cell medical industry is set for broader growth prospects, offering new hope for treating refractory diseases. Personalized therapy, based on individual patient characteristics, is becoming a key trend in the industry, with customized treatment plans likely to dominate future stem cell therapies. Technological innovation and breakthroughs will further drive industry growth, providing new strategies for severe chronic diseases.
How to obtain the latest research advancements in the field of biopharmaceuticals?
In the Synapse database, you can keep abreast of the latest research and development advances in drugs, targets, indications, organizations, etc., anywhere and anytime, on a daily or weekly basis. Click on the image below to embark on a brand new journey of drug discovery!
Refrence
- 1.Yamanaka S. Pluripotent Stem Cell-Based Cell Therapy-Promise and Challenges. Cell Stem Cell. 2020 Oct 1;27(4):523-531. doi: 10.1016/j.stem.2020.09.014. PMID: 33007237.
- 2.Tewary M, Shakiba N, Zandstra PW. Stem cell bioengineering: building from stem cell biology. Nat Rev Genet. 2018 Oct;19(10):595-614. doi: 10.1038/s41576-018-0040-z. PMID: 30089805.
- 3.Induced pluripotent stem cells (iPSCs): molecular mechanisms of induction and applications.