Advancing Diabetes Treatment: The Approval and Potential of Bexagliflozin in the Global Pharmaceutical Market
Bexagliflozin is a small molecule drug designed to target SGLT2 and is primarily used in the therapeutic areas of endocrinology and metabolic disease, specifically for the treatment of Diabetes Mellitus, Type 2. The originator organization of Bexagliflozin is Theracos, Inc. As of the latest available data, Bexagliflozin has reached its highest phase of development in the global market, receiving approval for use. In China, the drug is currently in the NDA/BLA phase. The first approval for Bexagliflozin was granted on January 1, 2023, in the United States. These factors indicate that Bexagliflozin has successfully completed the necessary clinical trials and regulatory processes to attain approval for its specified indications in the United States.
Due to the target and therapeutic areas of Bexagliflozin, it is intended to address the needs of patients with Diabetes Mellitus, Type 2, offering potential benefits in managing the condition. The approval of Bexagliflozin in the United States signifies a significant milestone for the drug, as it demonstrates its safety, efficacy, and compliance with regulatory standards in a major pharmaceutical market.
Below, we will use the drug Bexagliflozin as an example to demonstrate how to quickly obtain information about its chemical structure and patent situation using the Patsnap Chemical.
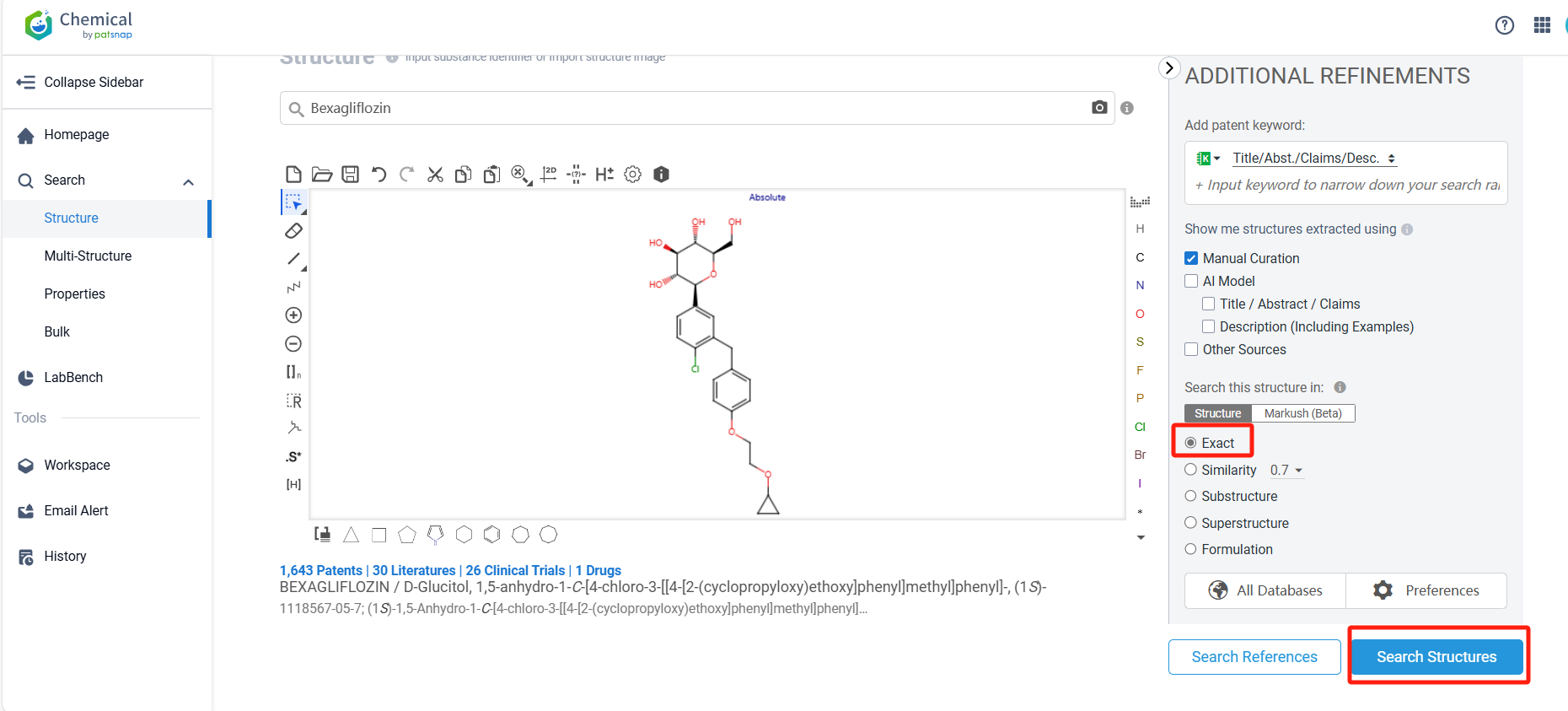
Log in to the Patsnap Chemical. Select the structural search and enter the common identity information of Bexagliflozin (such as CAS number, generic substance name, molecular formula, SMILES file, etc.). Here, we select "Exact Search", click on search structures, and you can find 57 patents. In the sidebar, select "Formulation" and "Use" under the "Claim Types" to search for patents related to new formulations and new indications. Clicking the "view in Analytics" will direct you to the Patsnap Patent.
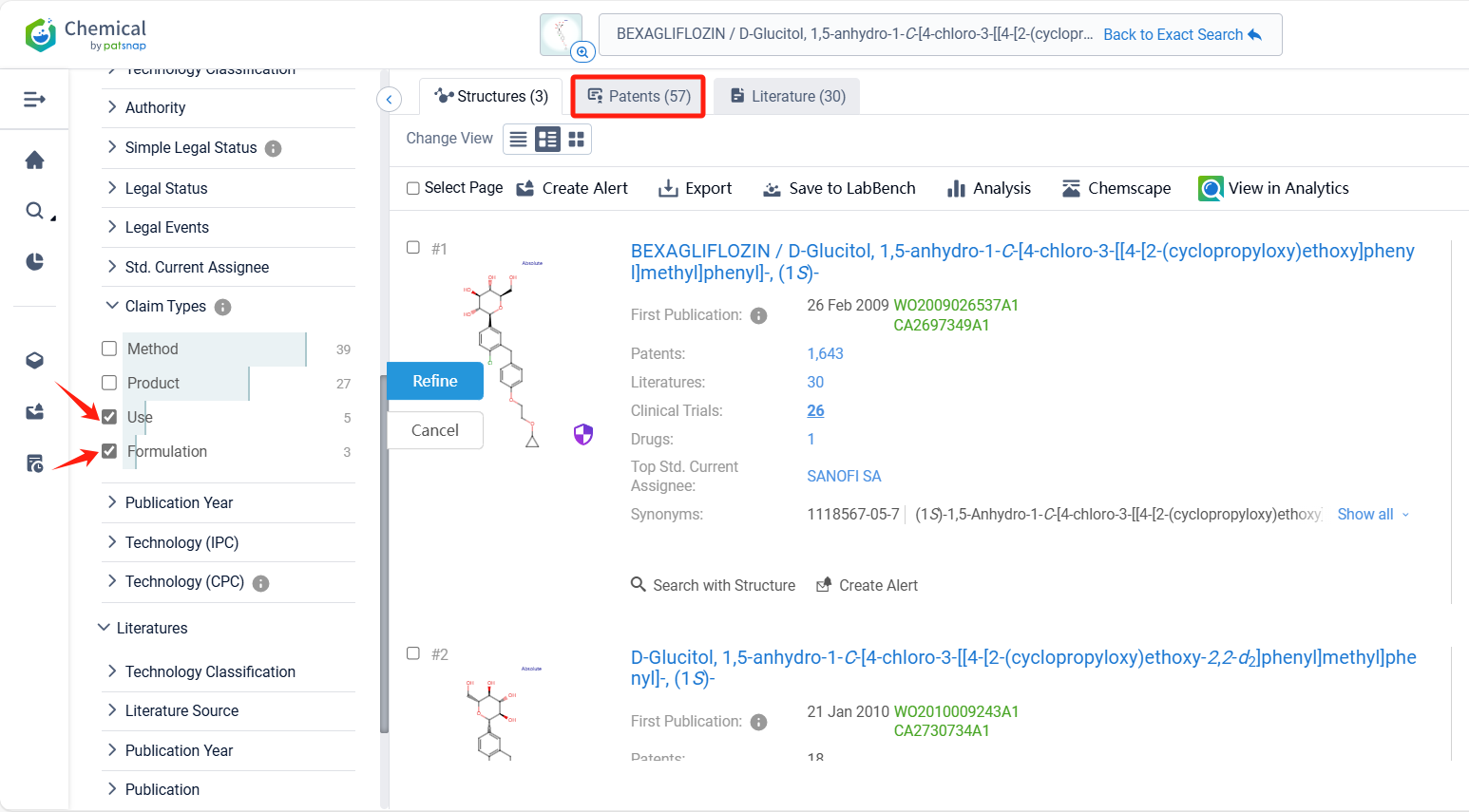
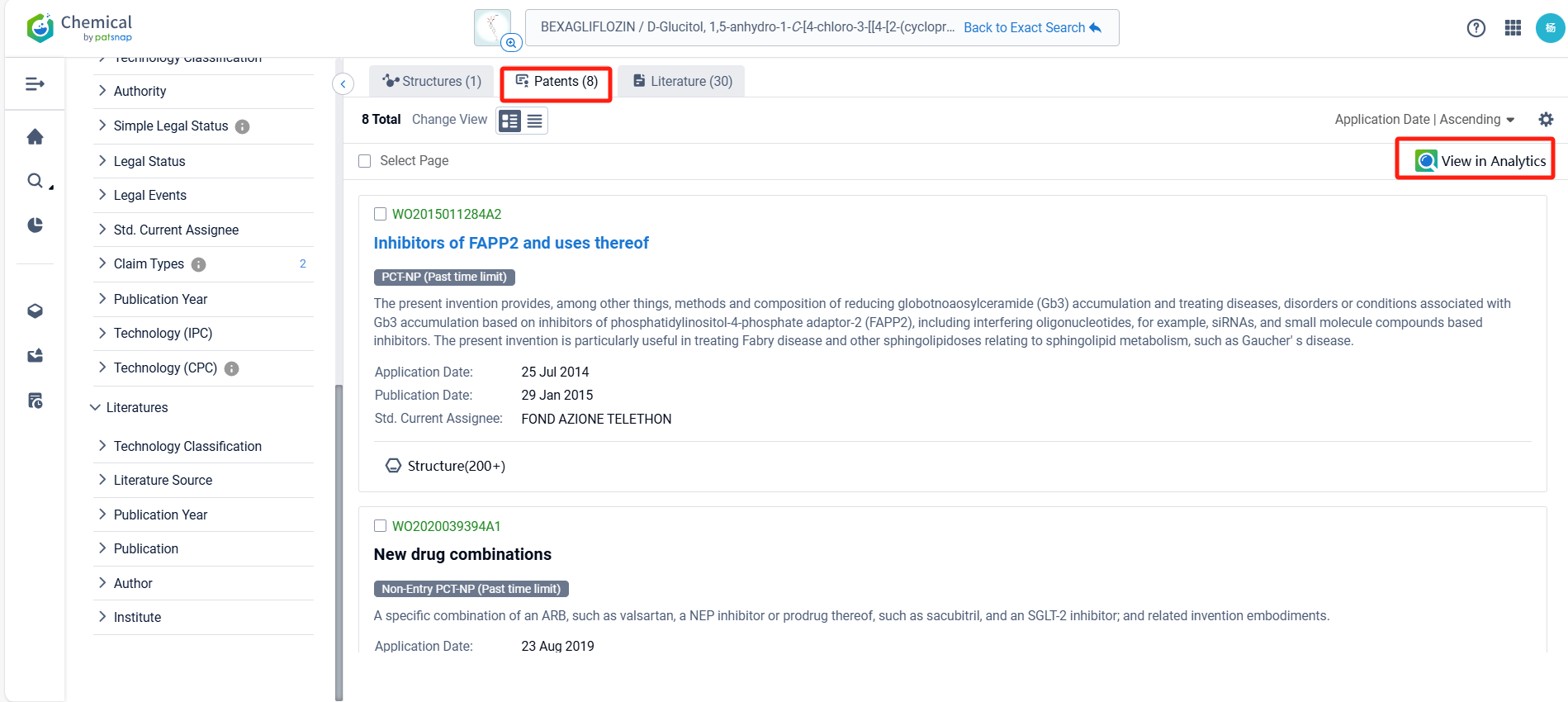
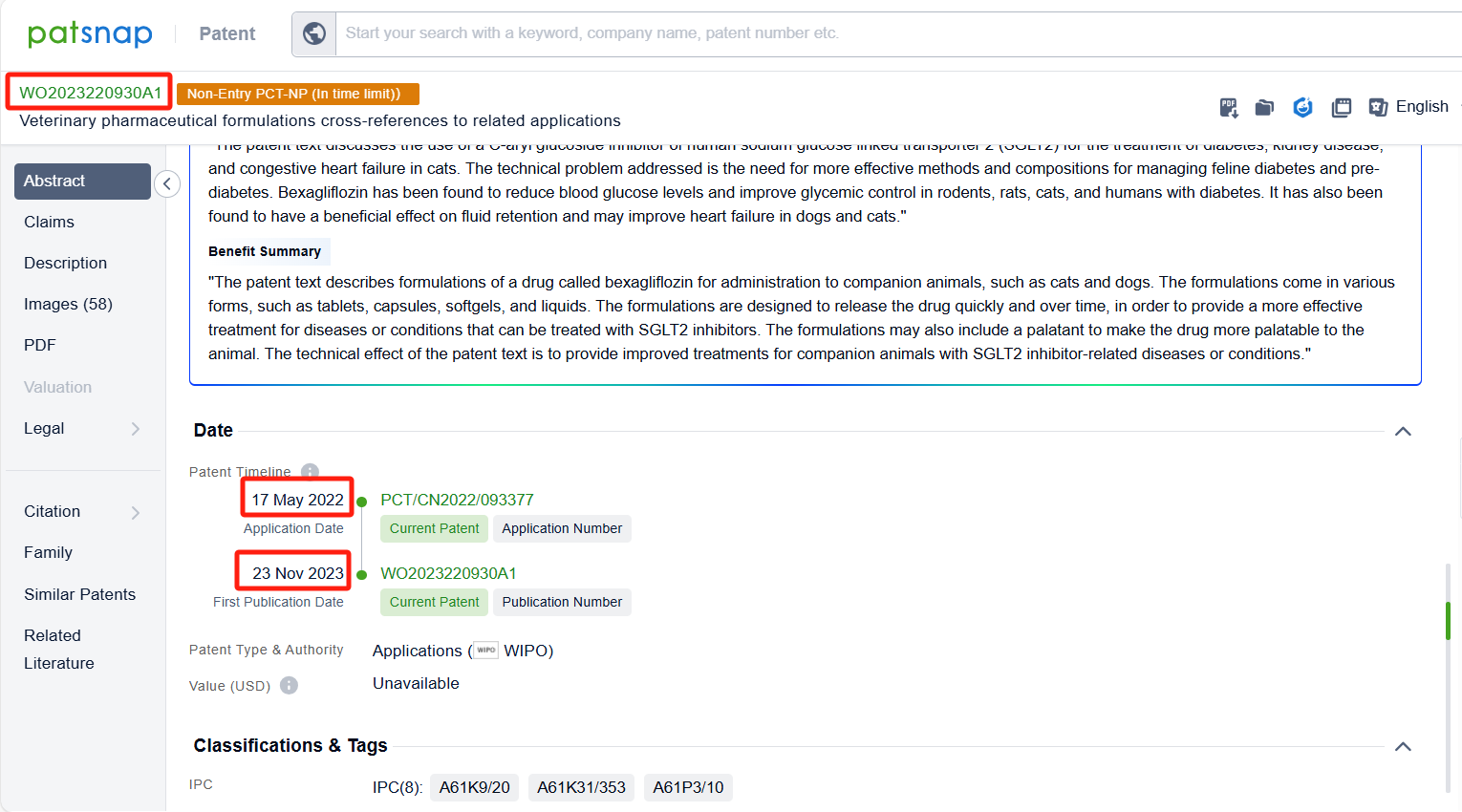
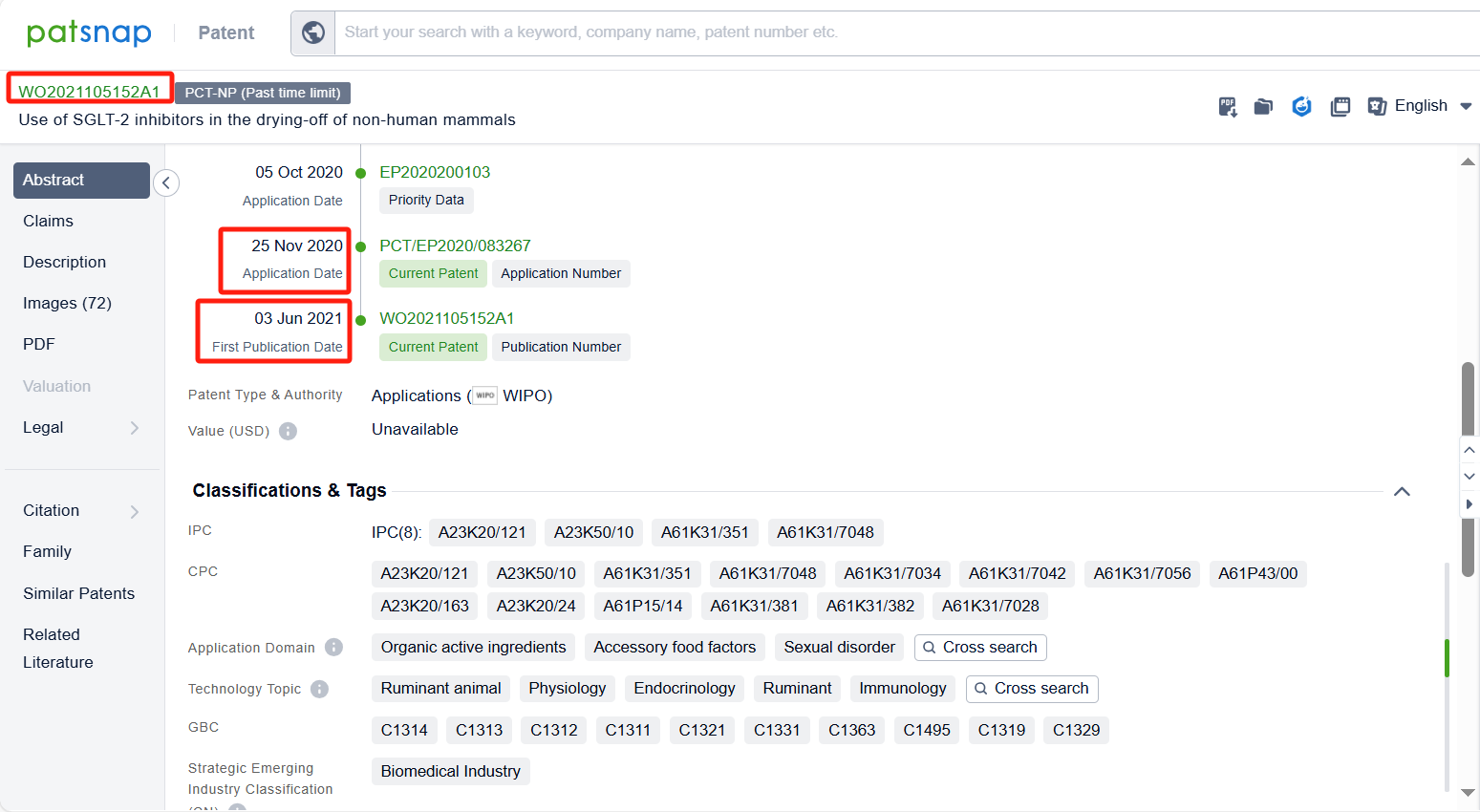
In the Patsnap patent database, we can sort patents by their publication dates to identify the latest patents on Bexagliflozin. By reviewing the aforementioned patents, we can observe that IncreVet, Inc.'s international patent WO2023220930A1 (application date 20220517, publication date 20231123) describes formulations of a drug called bexagliflozin for administration to companion animals, such as cats and dogs. Additionally, Boehringer Ingelheim Vetmedica Gmbh's patent WO2021105152A1 (application date 20201125, publication date 20210603) relates to the use of an SGLT-2 inhibitor for the treatment of non-human mammals, particularly ruminants, for various purposes such as drying-off, reducing milk production and discomfort associated with udder engorgement.
Business Development professionals in the pharmaceutical industry should pay close attention to Bexagliflozin, considering its potential for addressing unmet medical needs in the field of endocrinology and metabolic disease. Monitoring the progress of Bexagliflozin's regulatory status in China and other key markets will be crucial to understanding its global market potential and opportunities for further development and commercialization.
In conclusion, the approval of Bexagliflozin in the United States marks an important achievement for Theracos, Inc. and presents opportunities for the drug's potential impact on the treatment of Diabetes Mellitus, Type 2. As the drug progresses through subsequent regulatory phases and potential market expansion, it is essential for professionals in the pharmaceutical industry to closely follow its developments and consider potential business opportunities associated with Bexagliflozin.
AI built to maximize IP and R&D efficiency
Redefine chemical FTO with a range of structure retrieval options at your fingertips, from exact matches to similarity searches, all powered by deep data processing techniques and proprietary AI algorithms to eliminate the risk of omitting key results.