Agenus is set to secure a $25 million achievement fee from BMS for their collaborative TIGIT-CD96 dual targeting project
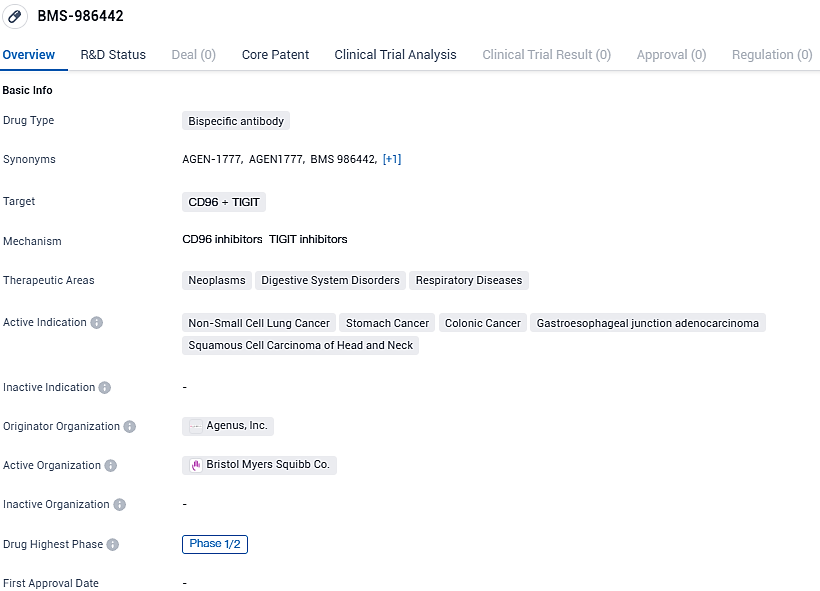
Agenus Inc., a pioneer in pioneering innovative agents that modulate the immune system for cancer treatment, has revealed that it has achieved the second developmental milestone under its worldwide licensing contract with Bristol Myers Squibb. This milestone pertains to BMS-986442, a bispecific TIGIT antibody with Fc enhancement features.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Agenus is set to receive a financial boost as Bristol Myers Squibb prepares to transfer $25 million in funds following the initiation of treatment for the initial enrollee in Phase 2’s dose expansion segment within the continuous CA115-001 study for BMS-986442.
BMS-986442, also identified as AGEN1777, targets both TIGIT and CD96 as an antagonist, and is designed with a potentiated Fc region to strengthen the reaction of T cells against tumors. The agreement for Bristol Myers Squibb to gain access to BMS-986442 from Agenus took place in 2021. With the completion of the Phase 1 dose escalation in solid tumor subjects, the ongoing study now evaluates the efficacy of BMS-986442 in conjunction with nivolumab, with or without concurrent chemotherapy application, in the Phase 2 expansion of dosage.
Garo Armen, Ph.D., and CEO, shared his enthusiasm about the progression into the Phase 2 segment of the dose expansion study, emphasizing the significance of this anti-TIGIT initiative and its potential impact on providing a new therapeutic avenue for individuals battling cancer.
Moreover, the arrangement encompasses the potential for Agenus to earn up to $1.32 billion based on future developmental and commercial achievements, alongside royalties ranging from high single-digits to mid-teens. Bristol Myers Squibb retains full responsibility for both the development and potential market release of BMS-986442 globally.
Agenus maintains certain preferential rights within the partnership, which include the possibility of undertaking additional clinical trials as per the developmental blueprint, the conduct of studies integrating specific other assets from the Agenus pipeline, the option for financial contribution to global development in exchange for higher royalties within the U.S., and the right to co-market BMS-986442 in the United States following its release.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
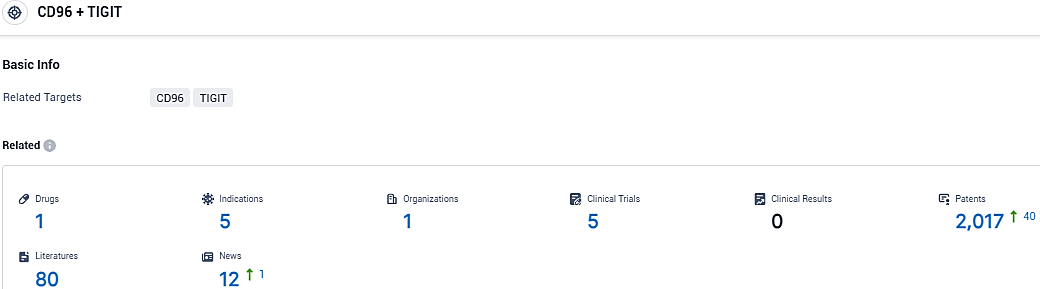
According to the data provided by the Synapse Database, As of December 16, 2023, there are 1 investigational drugs for the TIGIT and CD96 target, including 5 indications, 1 R&D institutions involved, with related clinical trials reaching 5, and as many as 2017 patents.
BMS-986442 targets CD96 and TIGIT proteins and is being investigated for its potential in treating various types of cancer, including non-small cell lung cancer, stomach cancer, colonic cancer, gastroesophageal junction adenocarcinoma, and squamous cell carcinoma of the head and neck. The drug is currently in Phase 1/2 of clinical trials, and further research is needed to determine its safety and efficacy in larger patient populations.