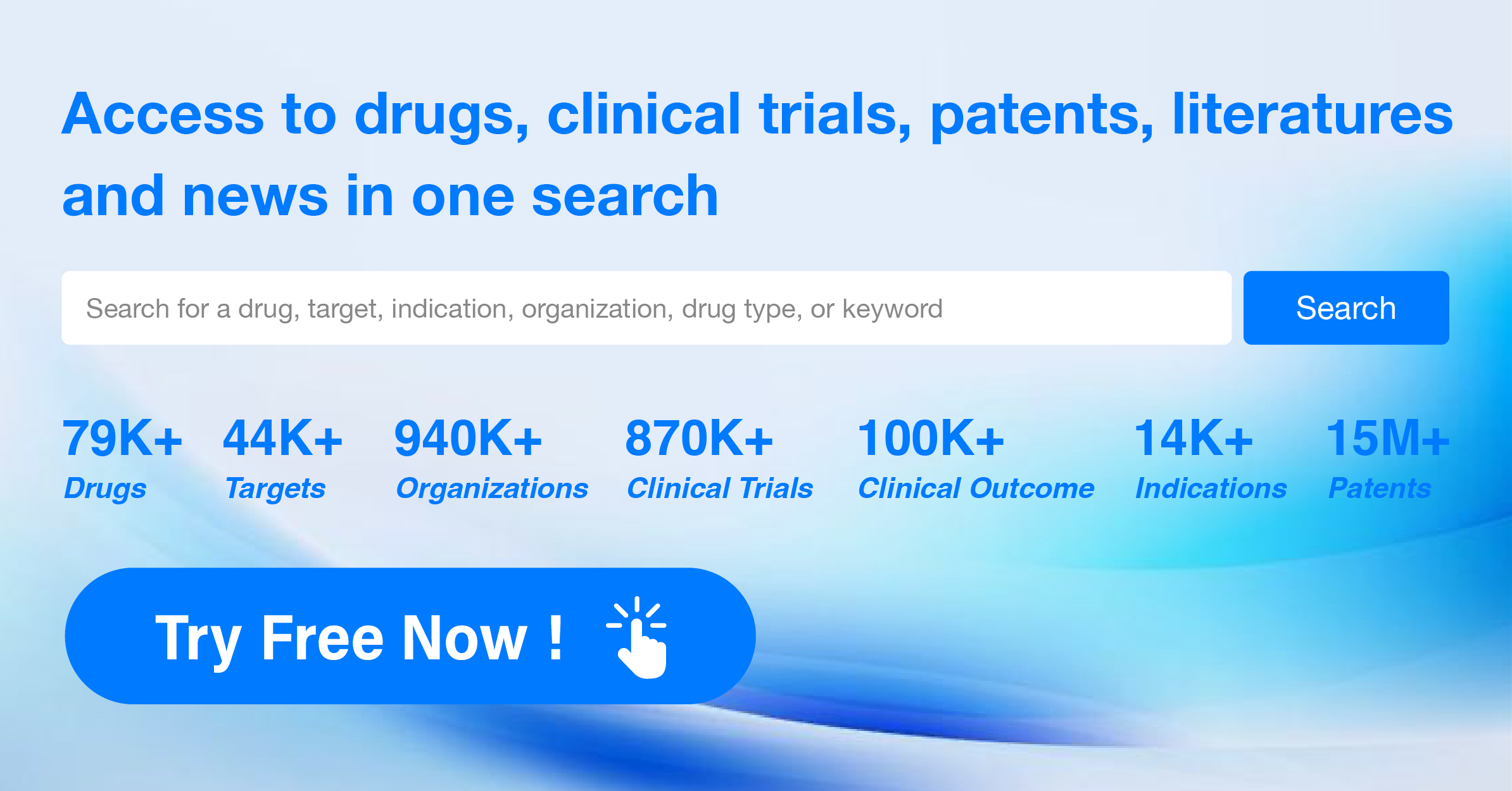
How to express the incidence of adverse reactions?
The incidence of adverse reactions recommended by the International Committee for the organization of Medical Sciences (CIOMS) is expressed as: very common (≥ 10%), common (1% - 10%, including 1%), occasional (0.1% - 1%, including 0.1%), rare (0.01% - 0.1%, including 0.01%), very rare (< 0.01%). The inducing factors of adverse drug reactions include non drug factors and drug factors. The former includes age, sex, heredity, susceptibility, disease, etc; The latter includes the toxic and side effects of drugs, drug interactions and the influence of excipients. Therefore, the adverse reactions of the same drug may behave differently in patients of different ages, genders, races, constitutions, indications, and pathological states. In addition, the influence of excipients in drugs and their preparations makes the problem more complex, which is the reason for the unpredictable adverse reactions of drugs.