AiCuris Initiates Phase 1 Trial of AIC468 for BK Virus in Kidney Transplant Patients
AiCuris Anti-infective Cures AG has announced the launch of a Phase 1 first-in-human study for AIC468, an innovative antisense oligonucleotide. AIC468 serves as an antiviral compound designed to address BK virus (BKV) infections in recipients of kidney transplants (KT). The reactivation of BKV in these patients represents a significant unmet medical requirement, as there are currently no approved therapeutic options. This Phase 1 trial (2023-510074-13-00) will assess the safety, tolerability, and pharmacokinetics of AIC468 among healthy volunteers.
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
“BK virus infections pose a significant risk to transplant recipients, resulting in graft loss and serious complications,” stated Dr. Cynthia Wat, Chief Medical Officer of AiCuris. “Moving AIC468 into clinical trials takes us a step closer to offering a targeted therapy that specifically inhibits the replication of the BK virus, addressing this pressing unmet medical requirement. Our team is dedicated to enhancing outcomes for patients facing the challenges of viral infections following transplantation.”
“This clinical trial represents an important achievement in our goal to create innovative treatments for individuals with compromised immune systems, who are especially susceptible to severe viral infections, including BK virus,” remarked Larry Edwards, CEO of AiCuris. “The launch of this trial illustrates the robustness and variety of our therapeutic pipeline as we push forward with advanced solutions for high-risk patient groups. With several programs currently in the clinical stage, we are optimistic that our strategy can significantly improve the lives of those who are immunocompromised.”
This randomized, double-blind, placebo-controlled trial is the first in humans to evaluate the safety, tolerability, and pharmacokinetics of AIC468 in 72 healthy subjects. Taking place in Germany, the study will assess both single and multiple ascending doses of AIC468, with preliminary data from the single ascending dose phase anticipated in 2025.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
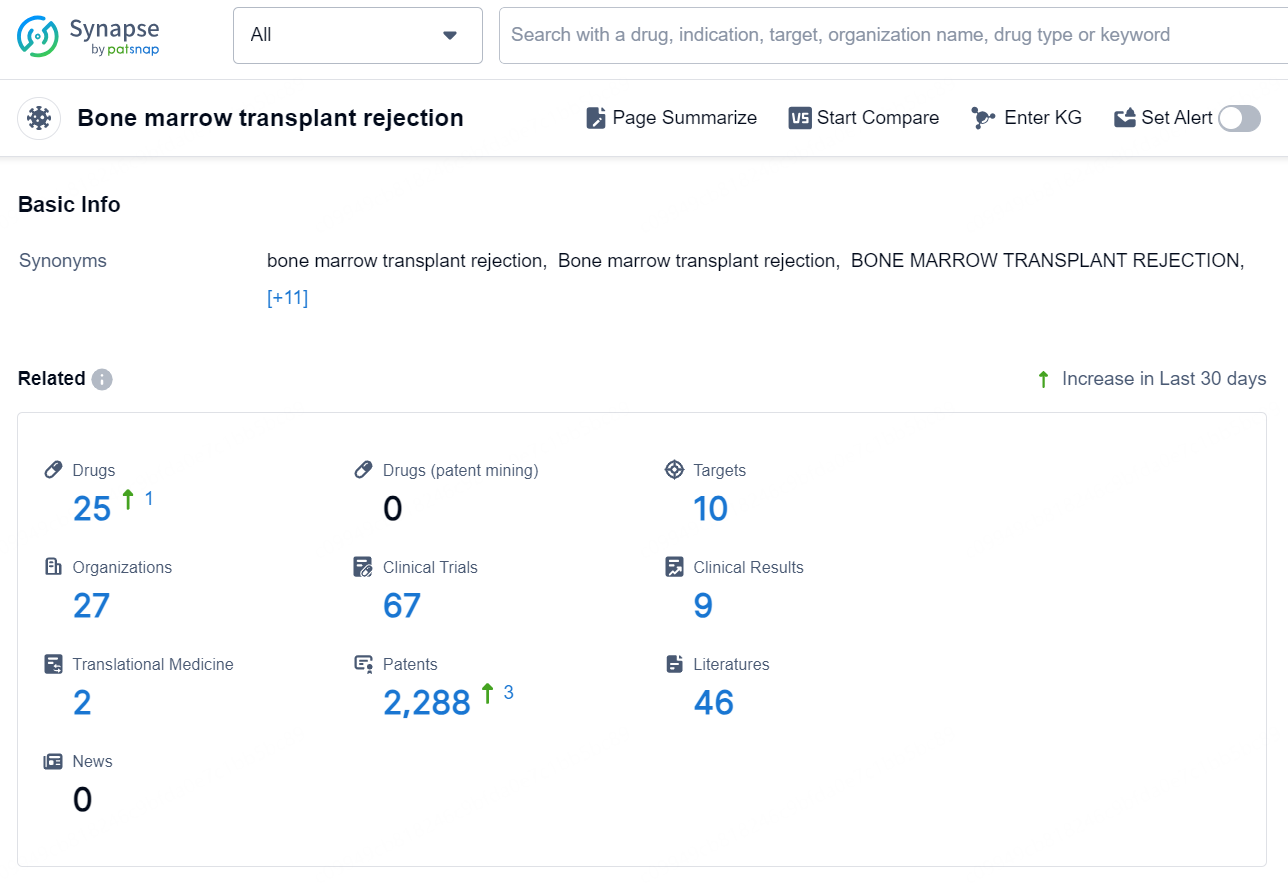
According to the data provided by the Synapse Database, As of November 5, 2024, there are 25 investigational drugs for the bone marrow transplant rejection, including 10 targets, 27 R&D institutions involved, with related clinical trials reaching 67, and as many as 2288 patents.
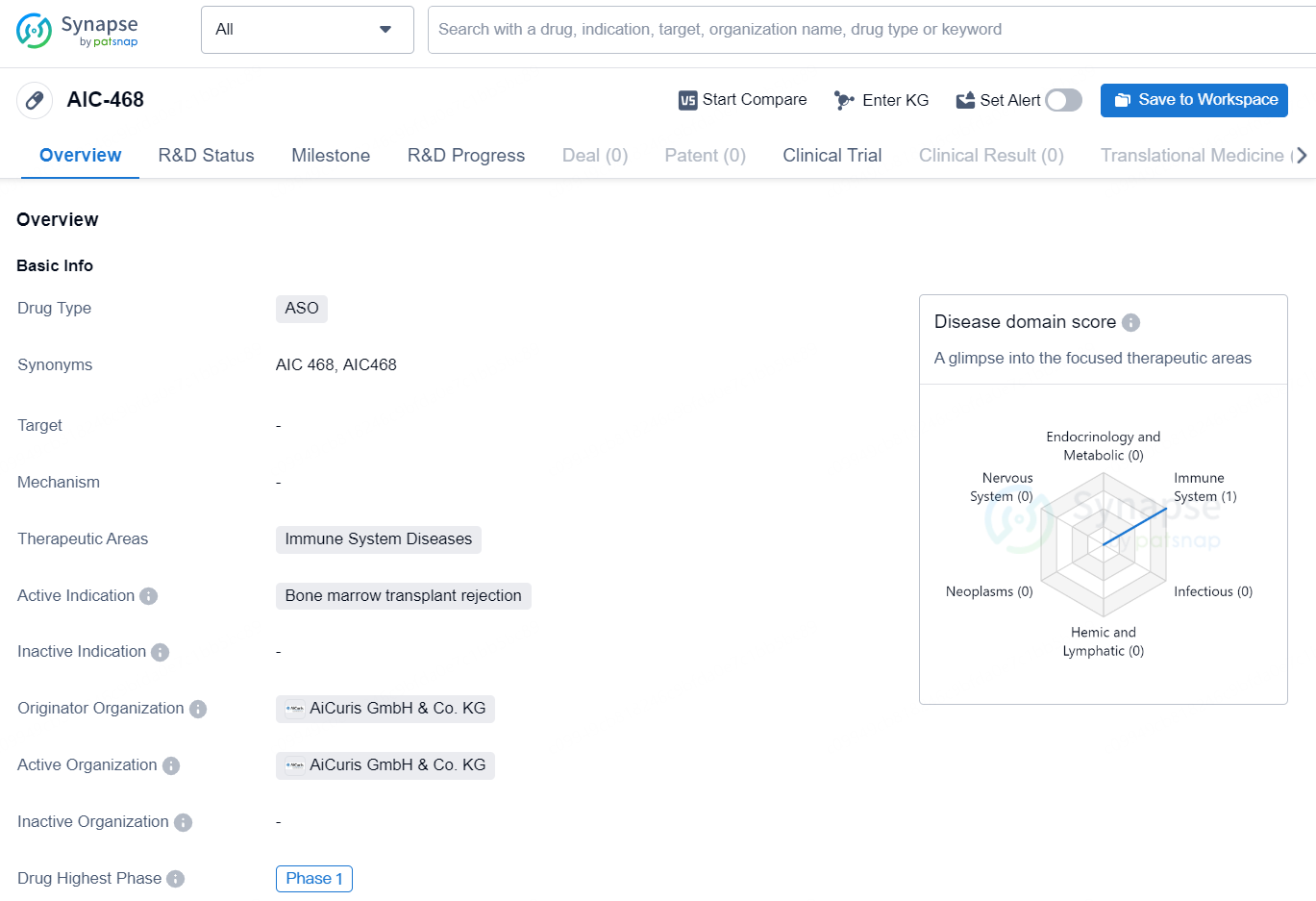
AIC-468 is an antisense oligonucleotide (ASO) drug being developed by AiCuris GmbH & Co. KG. The drug falls under the therapeutic area of immune system diseases and is being investigated for its potential to treat bone marrow transplant rejection. As an ASO drug, AIC-468 is designed to target specific RNA molecules involved in the rejection process, with the aim of modulating the immune response and preventing rejection of transplanted bone marrow. This approach holds promise for addressing the complex immune system dynamics involved in rejection and could potentially offer a more targeted and effective treatment option for patients undergoing bone marrow transplants.