Aldeyra Therapeutics Gets Full Feedback Note from U.S. FDA on New Eye Dryness Medication Submission, Reproxalap
Aldeyra Therapeutics, Inc., a firm specialized in biotech that focuses on the invention and advancement of novel treatments targeted at immune-related disorders, has disclosed that it has received a Complete Response Letter from the FDA regarding the New Drug Application of reproxalap. Reproxalap is an experimental pharmaceutical intended for the management of dry eye syndrome.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
While there were no specific concerns regarding safety or production with reproxalap, the FDA communicated through a letter that the New Drug Application (NDA) failed to convincingly show that the medication was effective in alleviating eye-related symptoms of dry eye disease. The FDA recommended that a new comprehensive, rigorously controlled trial should be conducted to confirm the positive impact of reproxalap on eye symptoms caused by dry eye.
It's projected that the review period for the Special Protocol Assessment (SPA) will be 45 days. Aldeyra aspires to receive the FDA's opinion on the SPA by December 2023. The estimated expense for the upcoming study is projected to be under $2 million, and interim data is expected to be available in the early part of 2024, pending input on the SPA from the FDA.
The timeline envisioned for submitting the NDA again is within the first two quarters of 2024, dependent on the feedback for the SPA and the success of the forthcoming trial. Aldeyra's strategy for the revamped NDA submission includes a preliminary product label that outlines both long-standing and instant symptomatic relief as well as an immediate decrease in eye redness due to reproxalap. The examination period for this updated NDA is anticipated to last half a year.
Todd C. Brady, M.D., Ph.D., president and CEO of Aldeyra Therapeutics, noted on September 30, 2023, that with $143 million in cash, cash equivalents, and marketable securities, the company is in a strong financial standing to proceed with another clinical study on reproxalap in dry eye disease patients, aiming for a NDA resubmission in early 2024.
Dr. Brady emphasized that, should the SPA and clinical study outcomes prove satisfactory and lead to an approved NDA resubmission, the medication's label could stand out in the dry eye disease market. It may be the inaugural label to detail both an immediate lessening of redness in the eyes and a blend of quick and extended symptom relief, underscoring reproxalap's swift effectiveness in treating both the physical indications and symptoms of dry eye disease.
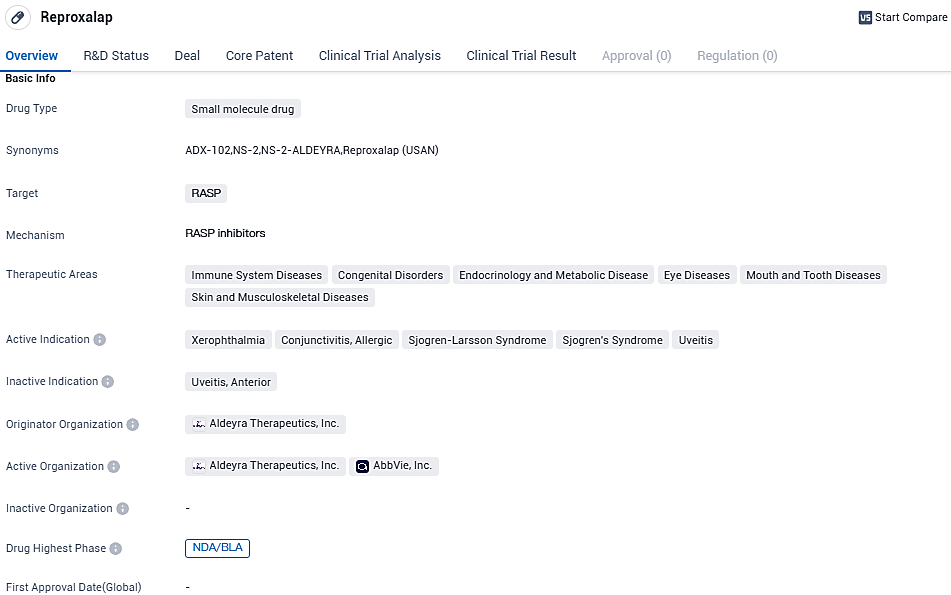
Reproxalap is being advanced as an experimental treatment option for dry eye disease and allergic conjunctivitis—two significant ophthalmological conditions. It operates as a novel RASP small-molecule inhibitor, with increased RASP levels found in both eye-related and systemic inflammatory conditions.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
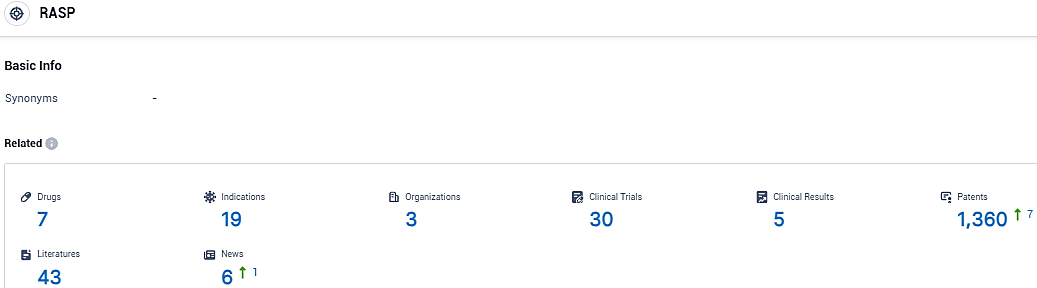
According to the data provided by the Synapse Database, As of December 5, 2023, there are 7 investigational drugs for the RASP target, including 19 indications, 3 R&D institutions involved, with related clinical trials reaching 30, and as many as 1360 patents.
Aldeyra is extending previous cash runway guidance into late 2025, including clinical trial costs associated with the proposed trial and potential NDA resubmission; the initial commercialization and launch plans for reproxalap, if approved in late 2024; and continued early and late-stage development of its product candidates in ocular and systemic immune-mediated diseases.