Neumora Therapeutics Reveals FDA Approval for NMRA-266 Investigational New Drug and Commences Phase 1 Human Trials
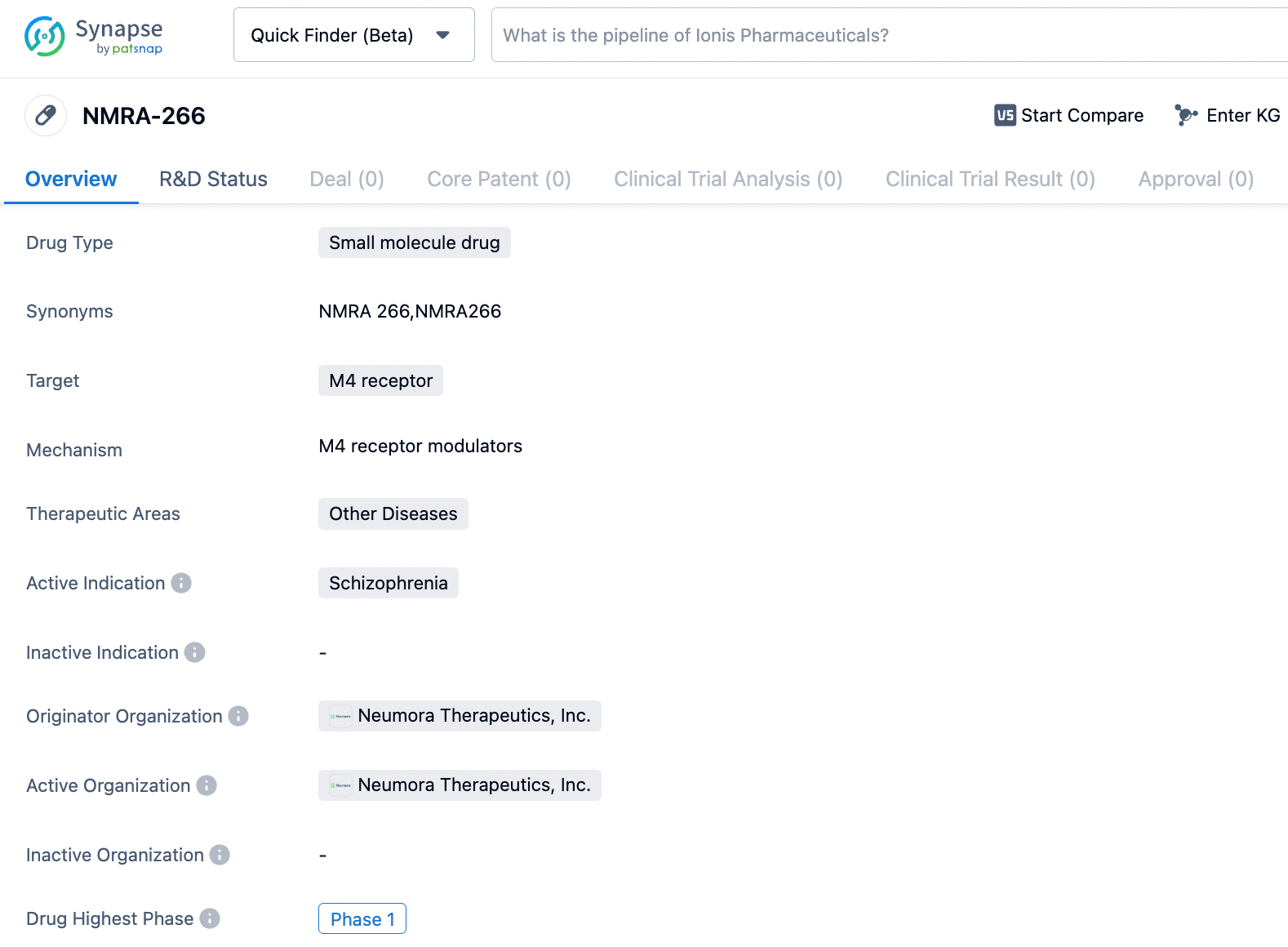
Neumora Therapeutics, Inc., an enterprise operating in the biopharmaceutical sector that is focusing on revolutionizing the creation of neuroscience medications, has disclosed the commencement of an early stage investigation under the designation Phase 1, involving the progressive increase of single and multiple dose administrations. This study aims to assess NMRA-266 in a group of healthy adult volunteers. NMRA-266, a molecule with selective affinity for enhancing the activity of the M4 muscarinic receptor, is being advanced by Neumora as a potential therapeutic option for the management of schizophrenia as well as various additional mental health conditions.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Embarking on the introductory phase of clinical evaluation marks a significant milestone for the progress of NMRA-266. As observed in preliminary laboratory assessments, NMRA-266 showcased an encouraging pharmacokinetic profile, notably its high efficacy and specificity in targeting the M4 receptor type, supporting its further progression to clinical trials,” expressed Robert Lenz, M.D. Ph.D., the senior vice president in charge of research and development at Neumora.
Dr. Lenz continued, “Given its promising characteristics gleaned from early studies and the clinical endorsement of the M4 muscarinic receptor category in managing schizophrenia, NMRA-266 emerges as a compelling candidate for the treatment of various mental health conditions.”
Neumora is optimistic about NMRA-266's prospects as a discriminating, M4 receptor-biased, positive allosteric modulator, projecting that it might offer therapeutic benefits in psychosis management akin to antipsychotics but with a lower incidence of typical side effects seen with wide-ranging muscarinic activators.
“Compounds with an affinity for muscarinic receptors have consistently shown significant effectiveness in many clinical settings, bolstering the credibility of this group of therapeutics as a strategy for addressing schizophrenia and other mental health anomalies,” stated John H. Krystal, M.D., the Robert L. McNeil, Jr. Professor of Translational Research, as well as a professor dedicated to Psychiatry, Neuroscience, and Psychology, and the Psychiatry Department Chair at the Yale School of Medicine.
Dr. Krystal elaborated, “Schizophrenia imposes severe impairments. Given the constraints of existing medication in terms of their effectiveness, there remains a profound need for improved treatment options. Consequently, the burgeoning pipeline of agents within the muscarinic category offers promise for individuals struggling with schizophrenia, potentially allowing them to find a therapeutic option that is effective for them.”
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
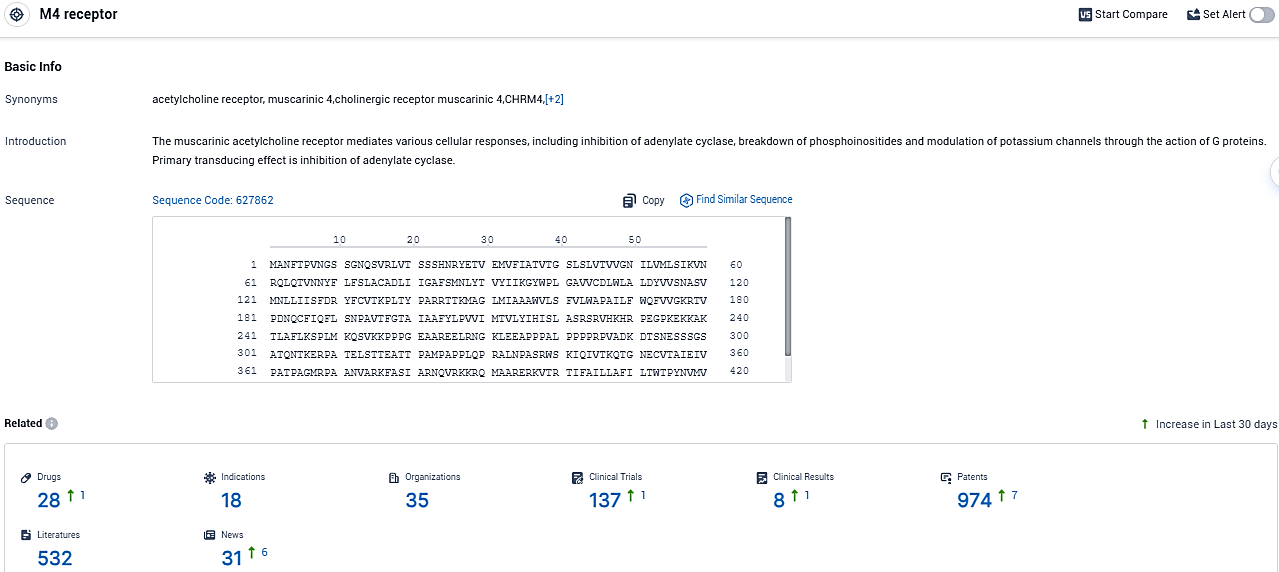
According to the data provided by the Synapse Database, As of December 5, 2023, there are 28 investigational drugs for the M4 receptor target, including 18 indications, 35 R&D institutions involved, with related clinical trials reaching 137, and as many as 974 patents.
NMRA-266 is an investigational positive allosteric modulator of the M4 muscarinic receptor subtype. M4 muscarinic receptor-targeting compounds have shown robust antipsychotic activity in multiple, placebo-controlled clinical trials, demonstrating potential as an approach to treating schizophrenia. Neumora exclusively licensed certain intellectual property rights related to NMRA-266 from Vanderbilt University, including composition of matter patent extending to 2042.