Alfasigma set to purchase Intercept Pharmaceuticals at $19.00 each stock in cash, broadening international reach in unusual and critical liver ailments
Alfasigma S.p.A, a prominent pharmaceutical enterprise in Italy, and Intercept Pharmaceuticals, Inc., a top-tier biopharmaceutical establishment specialising in uncommon and severe liver ailments, disclosed that they have established an unalterable merger contract. As per the agreement, Alfasigma has consented to purchase Intercept at a valuation of $19.00 per share, payable in cash. The planned deal is expected to significantly enhance Alfasigma’s gastrointestinal and hepatology collection and increase its business foothold in the U.S. market.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
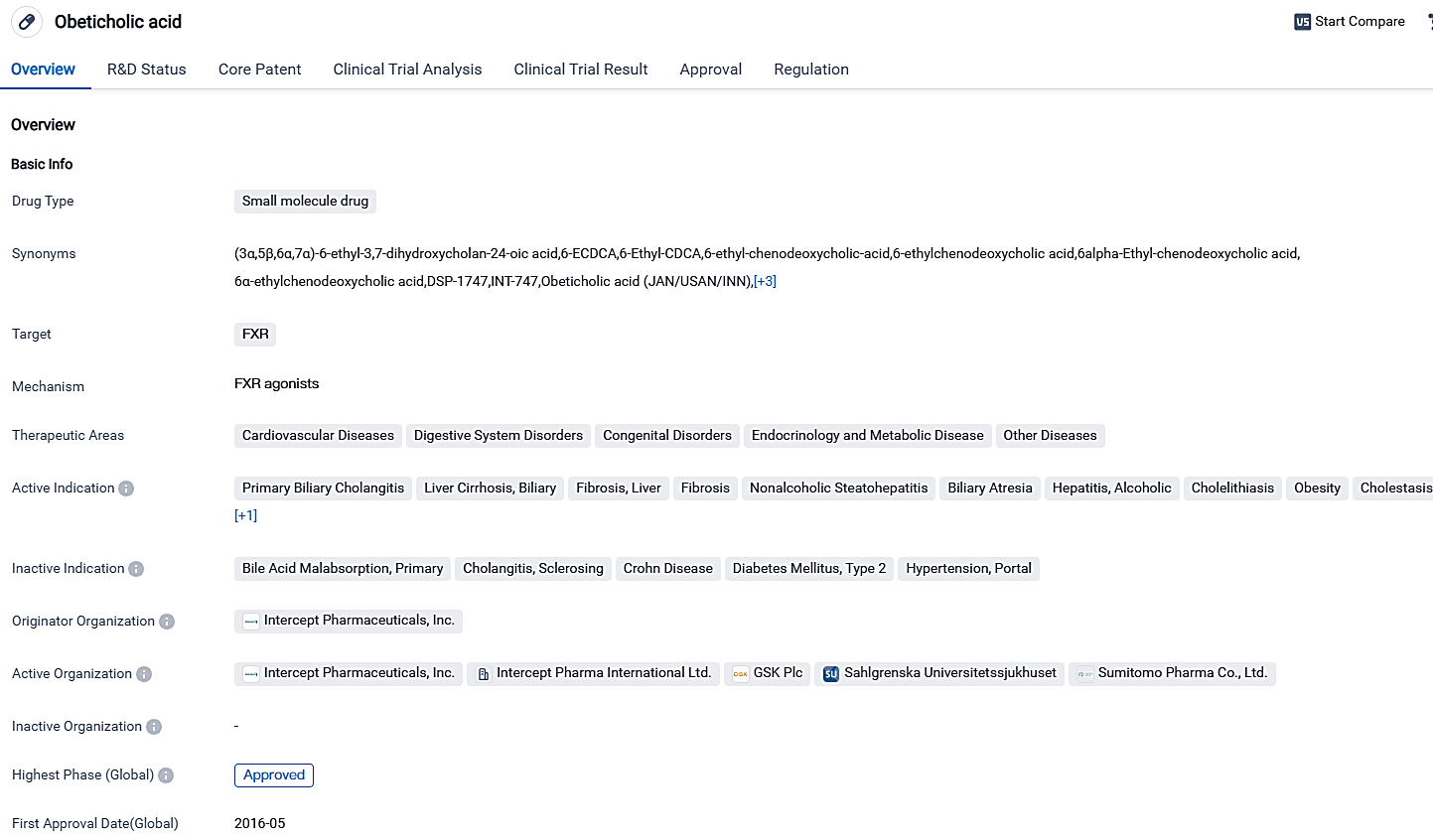
Intercept's principal drug is Ocaliva® (obeticholic acid), an FDA-approved farnesoid X receptor agonist in the United States and numerous other regions. It is used to treat primary biliary cholangitis (PBC) in adults unresponsive to UDCA, combined with ursodeoxycholic acid, or as a standalone treatment for adults who can't tolerate UDCA. Ocaliva® is the only approved second-line PBC therapy and its annual two-digit growth is supported by a skilled specialty sales team and a strong base of prescribers. Intercept further benefits from a wide clinical development pipeline led by a unique fixed dose combination of obeticholic acid and bezafibrate undergoing phase 2 trials for PBC.
"Strategically, the projected acquisition fits well with our aim to establish a stronger presence in the U.S. market, primarily focusing on our key gastroenterological area and enhancing our innovation pipeline with another significant asset," proclaimed Stefano Golinelli, Alfasigma Board's Chairman.
Francesco Balestrieri, Alfasigma's Chief Executive Officer, emphasized: “With the takeover of Intercept, Alfasigma has reached another significant goal in its growth journey, primarily in regards to the U.S. market where we have substantial expansion objectives. Intercept provides a compelling match for Alfasigma's core business areas like gastroenterology and hepatology. We are eager to welcome Intercept's team and anticipate a productive collaboration as we continue to invest in the company to unlock its full potential, benefiting patients.”
Jerry Durso, Intercept's President and Chief Executive Officer, shared his thoughts: “We are excited to make this transaction with Alfasigma public, which brings substantial advantages for our shareholders. We, as Intercept's team, are proud of the revolutionary work we have carried out, playing a pioneering role in delivering life-saving medicines to patients suffering from rare and serious liver diseases, such as PBC.”
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
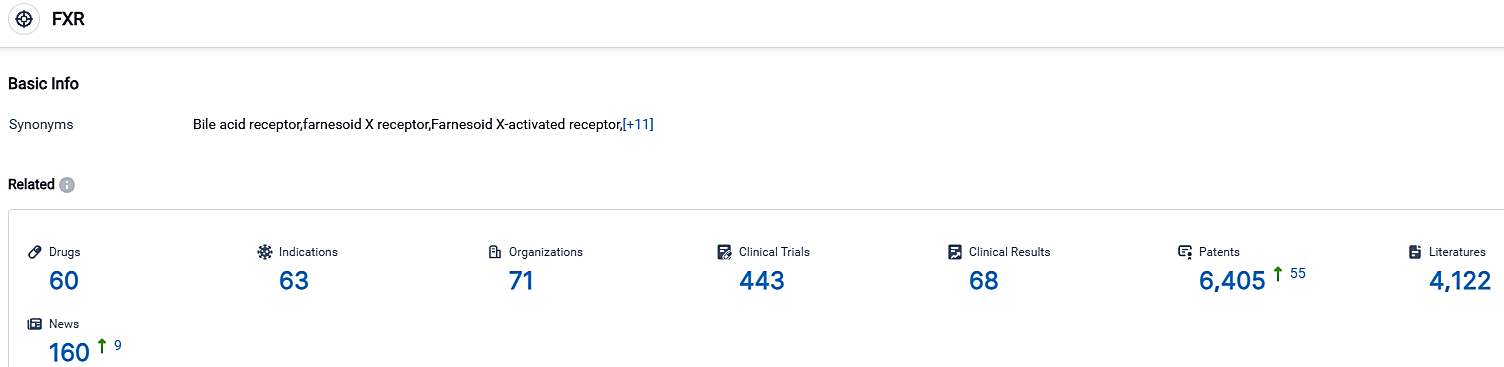
According to the data provided by the Synapse Database, As of October 3, 2023, there are 60 investigational drugs for the FXR target, including 63 indications,71 R&D institutions involved, with related clinical trials reaching 443,and as many as 6405 patents.
OCALIVA, acting as a farnesoid X receptor (FXR) agonist, is utilized for the treatment of adult individuals suffering from primary biliary cholangitis with balanced cirrhosis without any signs of portal hypertension. The drug was approved in the US while ceased in China at its most advanced stage, underlining the opportunities and hurdles in diverse markets. Moreover, the regulatory statuses of the medication underscore its significance in tackling prevalent unfulfilled healthcare requirements.