Alisertib Phase II Trial for Small Cell Lung Cancer Begins: ALISCA-Lung1
Puma Biotechnology, Inc., a company specializing in biopharmaceuticals, has recently declared the commencement of a Phase II study focusing on the use of ALISertib as a single-agent therapy. This clinical trial is designed to evaluate the efficacy of this treatment in individuals diagnosed with extensive stage small cell lung cancer.
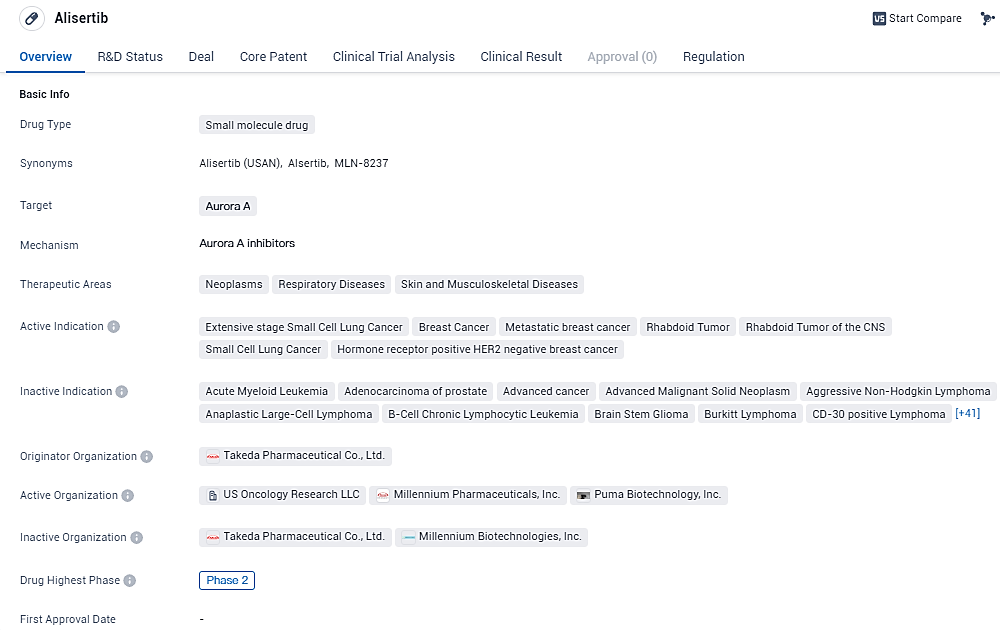
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
The ALISCA-Lung1 research is set to include a maximum of 60 individuals diagnosed with advanced-stage small cell lung cancer who have shown progression despite receiving initial platinum-based chemotherapy combined with immunotherapy treatments. Enrollment necessitates that participants submit tissue samples for the purpose of biomarker examination. The administration of Alisertib is prescribed at a rate of 50 mg BID from the 1st to the 7th day within each 21-day treatment cycle.
The focus of this study is to measure the objective response rate as the main goal, while also monitoring additional outcomes such as the length of the response, rate of disease control, time of progression-free survival, and total survival rates. Additionally, Puma is set to assess these outcomes relating to subsets of patients identified by specific pre-selected biomarkers to determine if increased effectiveness is observable within any of these subgroups.
In tandem with the ongoing clinical study, Puma is executing its biomarker analysis for the ALISCA-Lung1 endeavor. There are plans afoot to conduct a preliminary interim assessment that will scrutinize both the biomarkers and the therapeutic effectiveness. Depending on the research findings, Puma is hopeful of engaging with the U.S. Food and Drug Administration regarding the possibility of expedited approval for alisertib, particularly for the treatment of small cell lung cancer.
Alan H. Auerbach, holding the positions of CEO, President, and Founder at Puma, commented, "It brings us great satisfaction to proceed with this Phase II investigation. Our aspiration is that this study will shed considerable light on the therapeutic potential of alisertib for small cell lung cancer, and in an even more pinpointed manner, for those patients whose tumors exhibit specific molecular characteristics potentially susceptible to inhibition by alisertib, given its role as an aurora kinase A inhibitor."
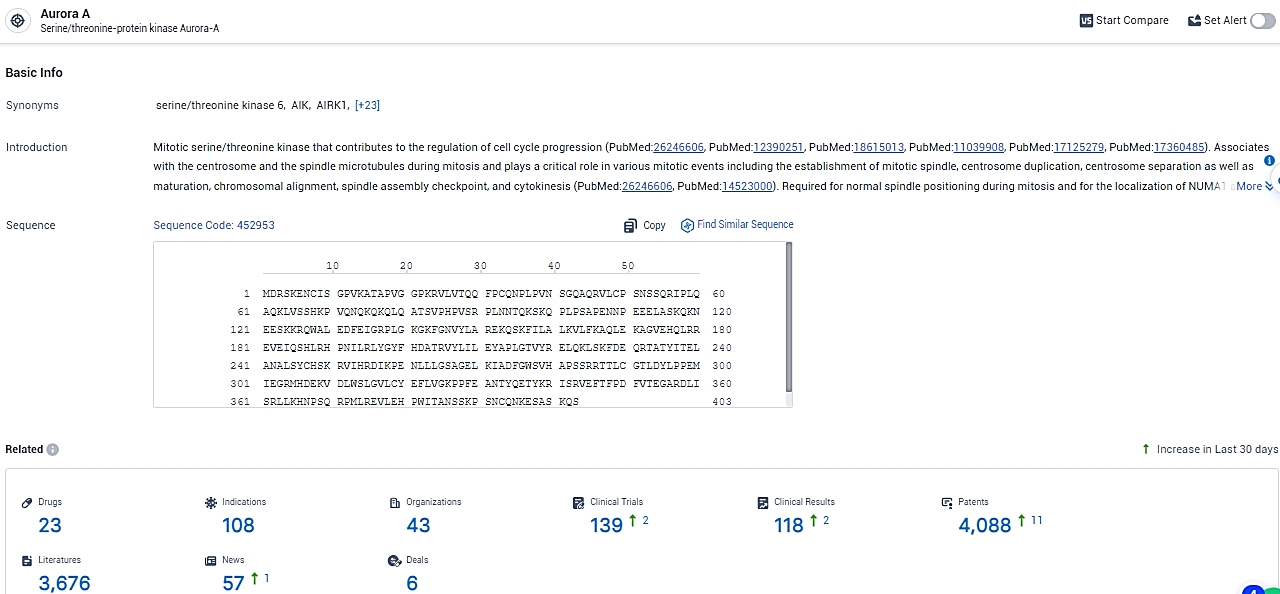
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of February 19, 2024, there are 23 investigational drugs for the Aurora A target, including 108 indications, 43 R&D institutions involved, with related clinical trials reaching 139, and as many as 4088 patents.
Alisertib targets Aurora A and is indicated for the treatment of extensive stage small cell lung cancer, various types of breast cancer, and rhabdoid tumor. Currently in Phase 2 of development, Alisertib holds the regulatory status of an Orphan Drug.