Alligator Bioscience Shows Promising Phase 2 Results from OPTIMIZE-1 Study Against Pancreatic Cancer
Alligator Bioscience has declared encouraging preliminary outcomes from its OPTIMIZE-1 Phase 2 trial examining mitazalimab, the company’s primary candidate, for the initial treatment of metastatic pancreatic cancer. Conducted as an open-label, multi-site investigation, this study evaluated the safety profile and effectiveness of the therapeutic combination involving mitazalimab (a CD40 monoclonal antibody agonist) and the conventional chemotherapy regimen mFOLFIRINOX in participants who had not undergone previous chemotherapy treatment.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
In the conducted research, the initial goal was met, revealing headline findings that indicated an established Objective Response Rate (ORR) of 40.4%, a preliminary ORR of 50.9%, and a rate of disease management of 79% among 57 assessable subjects, according to the established Response Evaluation Criteria in Solid Tumors. This outcome is notably better when compared to the ORR of 31.6% for a comparable cohort of patients who received only FOLFIRINOX.
The pivotal analysis was conducted on November 14, 2023, with a median monitoring period of 12.7 months. At the juncture of this examination, 29 participants were surviving, with 18 of them continuing the prescribed treatment. The maximum duration a participant continued therapy was recorded at 23 months, with three individuals achieving complete remission of their targeted lesions. The research further conveyed:
Alligator Bioscience's Chief Executive Officer, Søren Bregenholt, commented with enthusiasm, "The OPTIMIZE-1 trial has reached its primary goal, exhibiting that the pairing of mitazalimab with mFOLFIRINOX significantly extends survival in patients with pancreatic cancer, over the traditional treatment options."
He continued, "The remarkable length of the treatment's effect underscores the potent immune response incited by mitazalimab, leading to a notable enhancement in overall survival. Presently, the majority of the trial's participants are still active in the study, and we anticipate a continuous improvement in these initial positive outcomes."
Alligator Bioscience has actively engaged in strategic dialogues with the US Food and Drug Administration, outlining a definitive plan for mitazalimab's development and subsequent approval process in the treatment of pancreatic cancer. Drawing from the preliminary data of the OPTIMIZE-1 trial, the FDA has offered additional recommendations and recognized the study as one that facilitates the progression to a Phase 3 trial. Consequently, this has paved the way for mitazalimab to move straight into a global Phase 3 registration trial, which Alligator aims to commence at the start of 2025.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
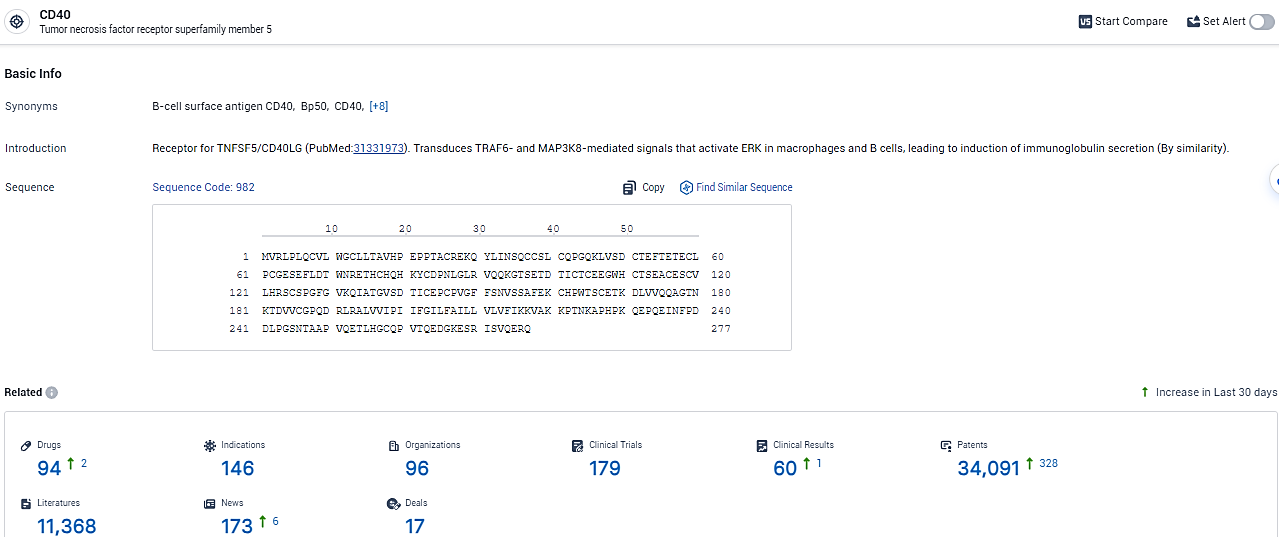
According to the data provided by the Synapse Database, As of February 2, 2024, there are 94 investigational drugs for the CD40 target, including 146 indications,96 R&D institutions involved, with related clinical trials reaching 179, and as many as 34091 patents.
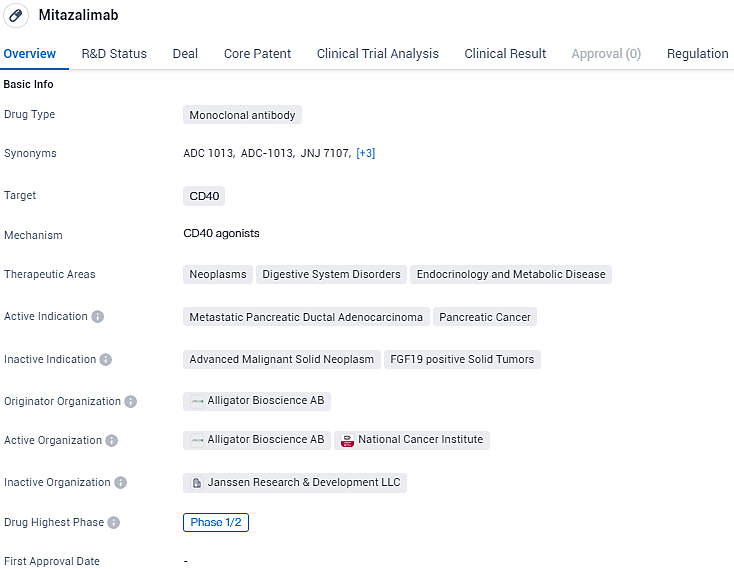
Mitazalimab is a monoclonal antibody drug developed by Alligator Bioscience AB that targets CD40. It is currently in Phase 1/2 of clinical development and shows promise in the treatment of metastatic pancreatic ductal adenocarcinoma. The drug also has potential applications in neoplasms, digestive system disorders, and endocrinology and metabolic diseases. Its orphan drug status highlights its potential to address an unmet medical need in the treatment of this rare form.