Altimmune Reveals Pemvidutide's Effects on Cardioinflammatory Lipids at ADA's 84th Session
Altimmune, Inc., a biopharmaceutical company in the clinical development phase, disclosed findings regarding the impact of pemvidutide—a GLP-1/glucagon dual receptor agonist being developed for treating obesity and metabolic dysfunction-associated steatohepatitis—on cardioinflammatory lipids at the American Diabetes Association’s 84th Scientific Sessions.
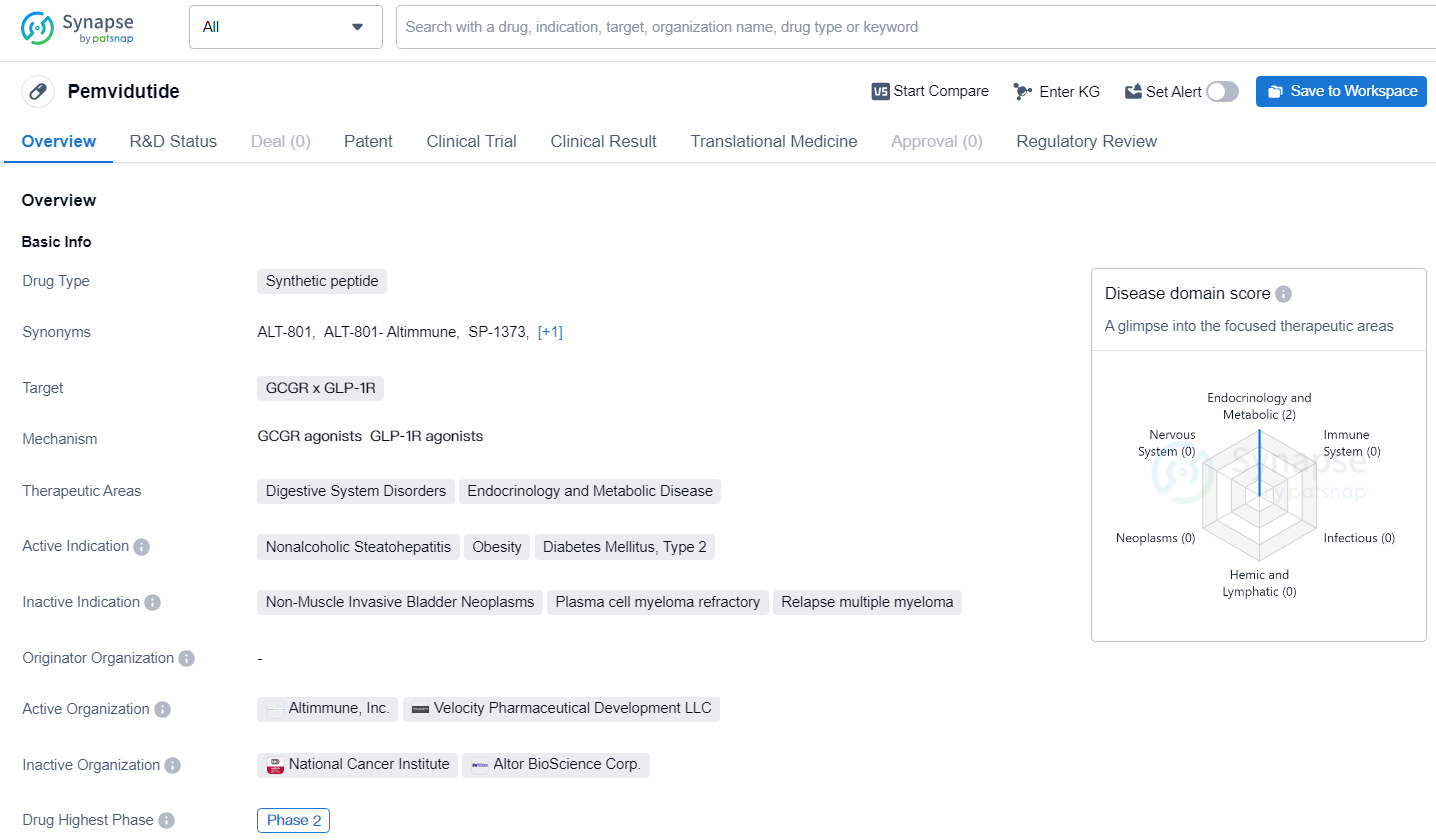
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
"Dyslipidemia is a prevalent and critical co-morbid condition associated with obesity, affecting up to 70% of individuals living with obesity," stated Vipin K. Garg, Ph.D., President and CEO of Altimmune. "These findings further support the unique profile of pemvidutide and emphasize its potential to diminish inflammatory lipids linked to cardiovascular plaque development and increased cardiovascular risk in obese patients."
Irregular lipid profiles in obese individuals can trigger systemic inflammation and heighten the risk of cardiovascular diseases. To gain a clearer understanding of pemvidutide's impact on biomarkers related to lipoproteins and glycoproteins involved in CVD inflammation, samples were examined from a 12-week, randomized, placebo-controlled Phase 1 trial of pemvidutide conducted on subjects with overweight or obesity, excluding those with type 2 diabetes. In this study, 34 participants were randomly divided into four groups in a 1:1:1:1 ratio to receive either pemvidutide or a placebo administered via subcutaneous injection once weekly for 12 weeks.
Lipidomic, lipoparticle, and glycoprotein profiling utilized ultra-high performance liquid chromatography-mass spectrometry and proton nuclear magnetic resonance on plasma samples, taken both at the study's onset and after 12 weeks of treatment.
Pemvidutide is an innovative, investigational peptide-based GLP-1/glucagon dual receptor agonist under development for obesity and MASH treatment. The activation of GLP-1 and glucagon receptors is thought to emulate the synergistic effects of diet and physical activity on weight reduction, with GLP-1 curbing appetite and glucagon boosting energy usage.
Glucagon is also acknowledged for its direct impact on hepatic fat metabolism, potentially leading to swift reductions in liver fat and serum lipids. In clinical studies so far, once-weekly pemvidutide has shown significant weight loss, notable decreases in triglycerides, LDL cholesterol, liver fat levels, and blood pressure. The U.S. FDA has given pemvidutide Fast Track designation for MASH treatment. Pemvidutide has concluded the MOMENTUM Phase 2 obesity trial and is currently being evaluated in the ongoing IMPACT Phase 2b MASH trial.
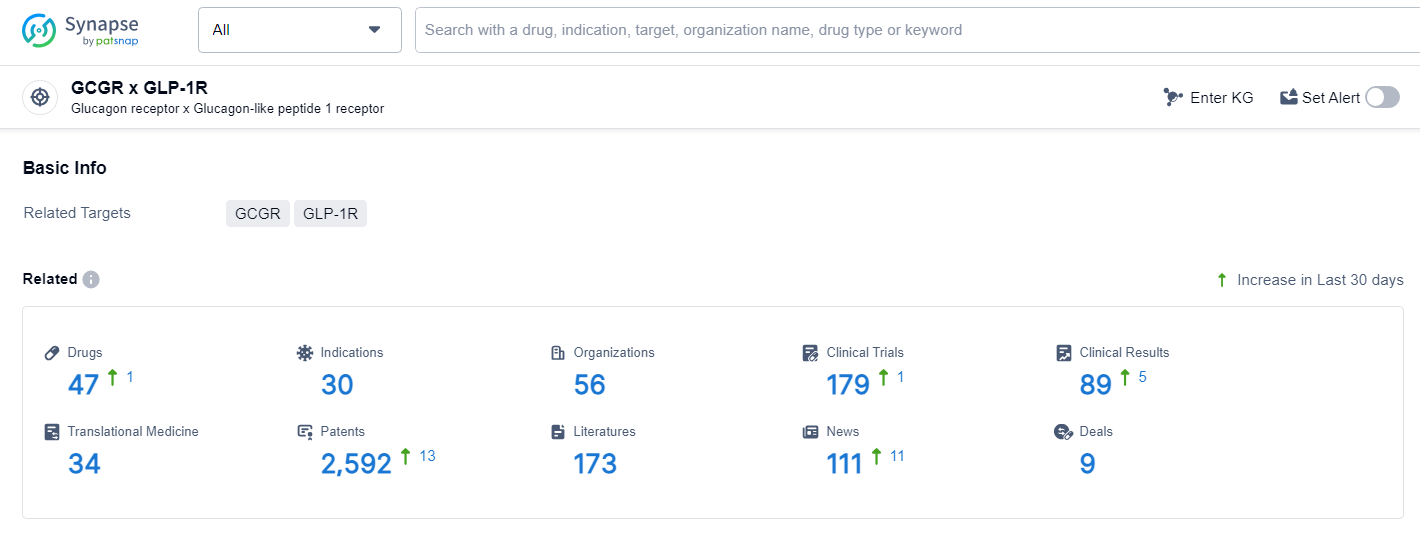
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of June 27, 2024, there are 47 investigational drugs for the GCGR and GLP-1R target, including 30 indications, 56 R&D institutions involved, with related clinical trials reaching 179, and as many as 2592 patents.
Pemvidutide has reached Phase 2 of development suggests that it has shown promise in early-stage clinical trials, prompting further investigation in a larger patient population. The Fast Track designation indicates that there is a recognized need for new treatment options in the therapeutic areas targeted by Pemvidutide, and the drug has the potential to address these unmet medical needs.