AltruBio Commences Initial Participant Recruitment for Second-Phase Clinical Trials of ALTB-268 Aimed at Managing Ulcerative Colitis
AltruBio Inc., a biotechnology firm focused on the advancement of innovative treatments targeting immunologic conditions lacking satisfactory medical solutions, has declared the initiation of its Phase 2a clinical trial with the inaugural enrollment of a participant. This study will investigate the effects of AltruBio's investigational drug, ALTB-268, which is delivered subcutaneously and acts as an immune checkpoint agonist, in individuals diagnosed with ulcerative colitis.
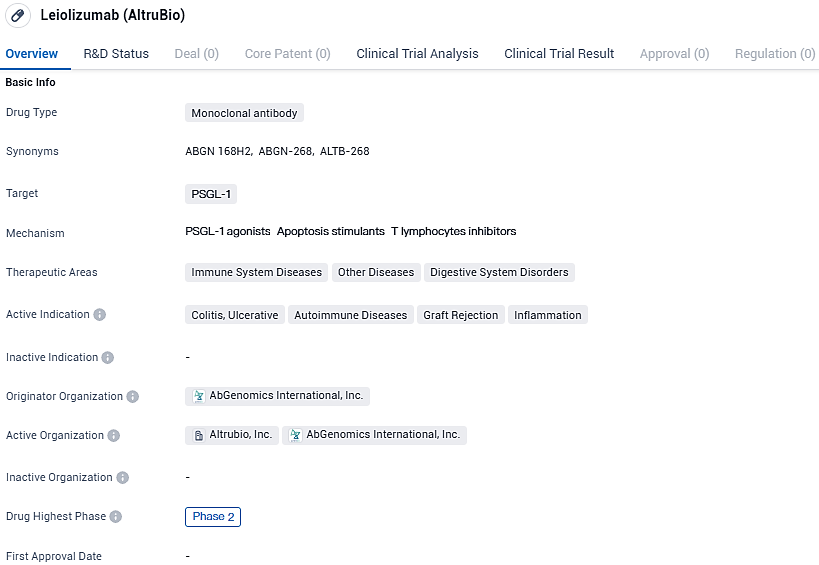
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Our firm's stage two clinical trials encompass a phase 2a non-blinded study targeting patients with biological therapy-resistant UC and a phase 2b study, which will be randomized and placebo-based, focusing on patients suffering from UC that ranges from moderate to severe in activity.
Dr. Jesse Hall, the Lead Medical Officer at AltruBio, expressed enthusiasm about achieving this pivotal point in their journey. "We are dedicated to getting ALTB-268 out to those dealing with autoimmune inflammatory conditions," he stated. "An increase in PSGL-1 has been noted across various autoimmune disorders, including UC. The intent of launching the Phase 2a trial is to pinpoint biomarkers that could potentially correlate with ALTB-268 administration. Our medication's unique way of interacting opens the door to an innovative treatment strategy for combatting inflammatory bowel disease."
Building on the phase 2a clinical study that's currently making headway, involving subjects with resistance to biologic treatments, there are plans in 2024 to embark on an extensive, international phase 2b trial. This randomized, placebo-controlled research will embrace subjects with UC activity levels ranging from moderate to severe. Importantly, it will welcome patients irrespective of their experience with advanced therapies, whether they are seasoned or not.
ALTB-268 functions as a quadrivalent PSGL-1 agonist antibody. Its role is to amplify immune checkpoints which selectively diminish the activity of persistently active T-cells by stunting their effector activities and fostering their exhaustion and programmed cell death.
ALTB-268's innovative approach is to recalibrate the immune system towards equilibrium, not through global suppression, but by targeting immune-related disorders at their core. Echoing the mechanism seen in its bivalent intravenous counterpart, ALTB-168, this drug has shown promise through its Phase 2 achievements in treating a range of conditions - ulcerative colitis, psoriasis, psoriatic arthritis, and steroid-refractory/therapy-resistant acute graft-versus-host diseases. The results have suggested enhancements in patient conditions and substantial therapeutic benefits.
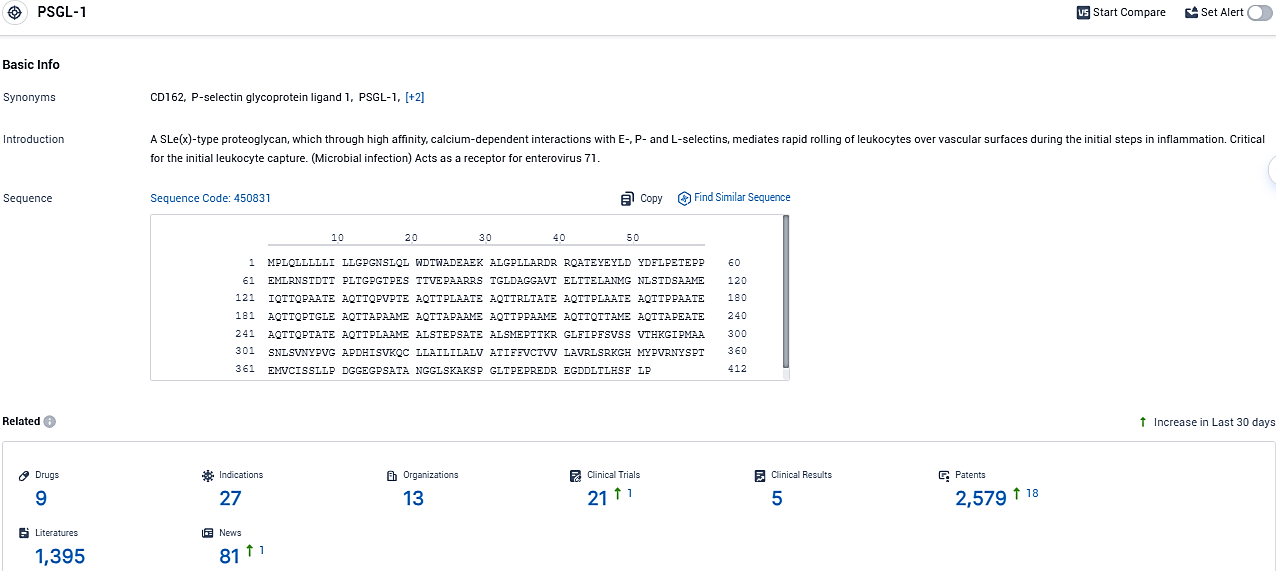
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of December 15, 2023, there are 9 investigational drugs for the PSGL-1 target, including 27 indications, 13 R&D institutions involved, with related clinical trials reaching 21, and as many as 2579 patents.
ALTB-268 shows promise as a potential treatment for immune system diseases, digestive system disorders, and other conditions. Its current status in Phase 2 clinical development indicates that it has progressed through initial safety testing and is now being evaluated for its efficacy and safety in a larger patient population. Further research and clinical trials will be necessary to determine the full potential and effectiveness of ALTB-268 in treating the indicated conditions.