Request Demo
Knowledge Base
Do adverse drug reactions have to have a clear causal relationship before they can be reported?
15 December 2023
2 min read
No. Determining the causal relationship between a drug and a suspected adverse reaction is sometimes very difficult and takes a long time. Generally, it can be reported as long as it is suspicious. Report adverse drug reactions that have occurred in a timely manner. The national drug regulatory department will analyze and evaluate the collected report data. Based on the evaluation results, timely measures can be taken to release information in various ways to limit or stop the production, sale and use of relevant drugs to avoid The recurrence of the same drugs and the same adverse reactions will protect the drug safety and health of more people, and even protect the safety and health of the next generation.
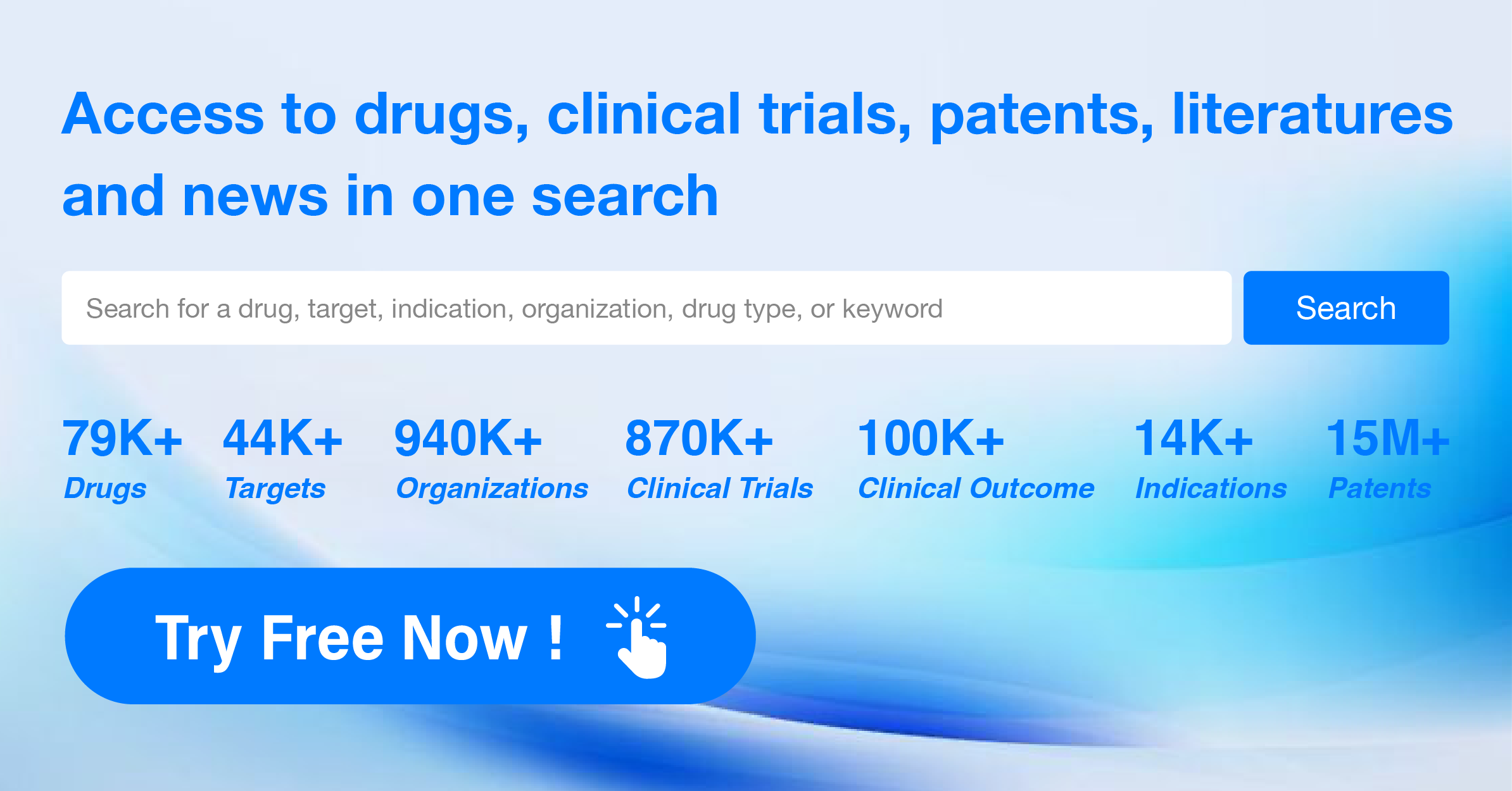
AI Agents Built for Biopharma Breakthroughs
Accelerate discovery. Empower decisions. Transform outcomes.
Get started for free today!
Accelerate Strategic R&D decision making with Synapse, PatSnap’s AI-powered Connected Innovation Intelligence Platform Built for Life Sciences Professionals.
Start your data trial now!
Synapse data is also accessible to external entities via APIs or data packages. Empower better decisions with the latest in pharmaceutical intelligence.
Keep Reading
Back to all blogs →Latest Hotspot
3 min read
US Health Agency Endorses Bio-Thera's Avzivi® as Alternative to Avastin®, Authorizing Bevacizumab-tnjn Use
15 December 2023
Bio-Thera Solutions, Ltd has made public the approval of their product Avzivi® (bevacizumab-tnjn) by the United States Food and Drug Administration.
Valuable Targets
5 min read
Understanding Plasminogen activators Inhibitors and Methods to Keep Abreast of Their Recent Developments
15 December 2023
Plasminogen activators inhibitors are substances that regulate clotting by inhibiting enzymes that break down blood clots.
Latest Hotspot
3 min read
ExeGi Pharma Reveals Initiation of Participant Inclusion for EXE-346 in the PROF Study
15 December 2023
ExeGi Pharma, a biopharmaceutical pioneer in live biotherapeutics, has commenced patient enrollment for its pivotal PROF Trial to test EXE-346, a new treatment targeting frequent bowel movements post ileal pouch-anal anastomosis surgery.
Knowledge Base
2 min read
What is the voluntary reporting for adverse drug reactions?
15 December 2023
After the "Thalidomide Incident" in the 1960s, regulatory authorities in many countries established voluntary reporting systems for adverse drug reactions to collect adverse drug reactions.
Get started for free today!
Accelerate Strategic R&D decision making with Synapse, PatSnap’s AI-powered Connected Innovation Intelligence Platform Built for Life Sciences Professionals.
Start your data trial now!
Synapse data is also accessible to external entities via APIs or data packages. Empower better decisions with the latest in pharmaceutical intelligence.